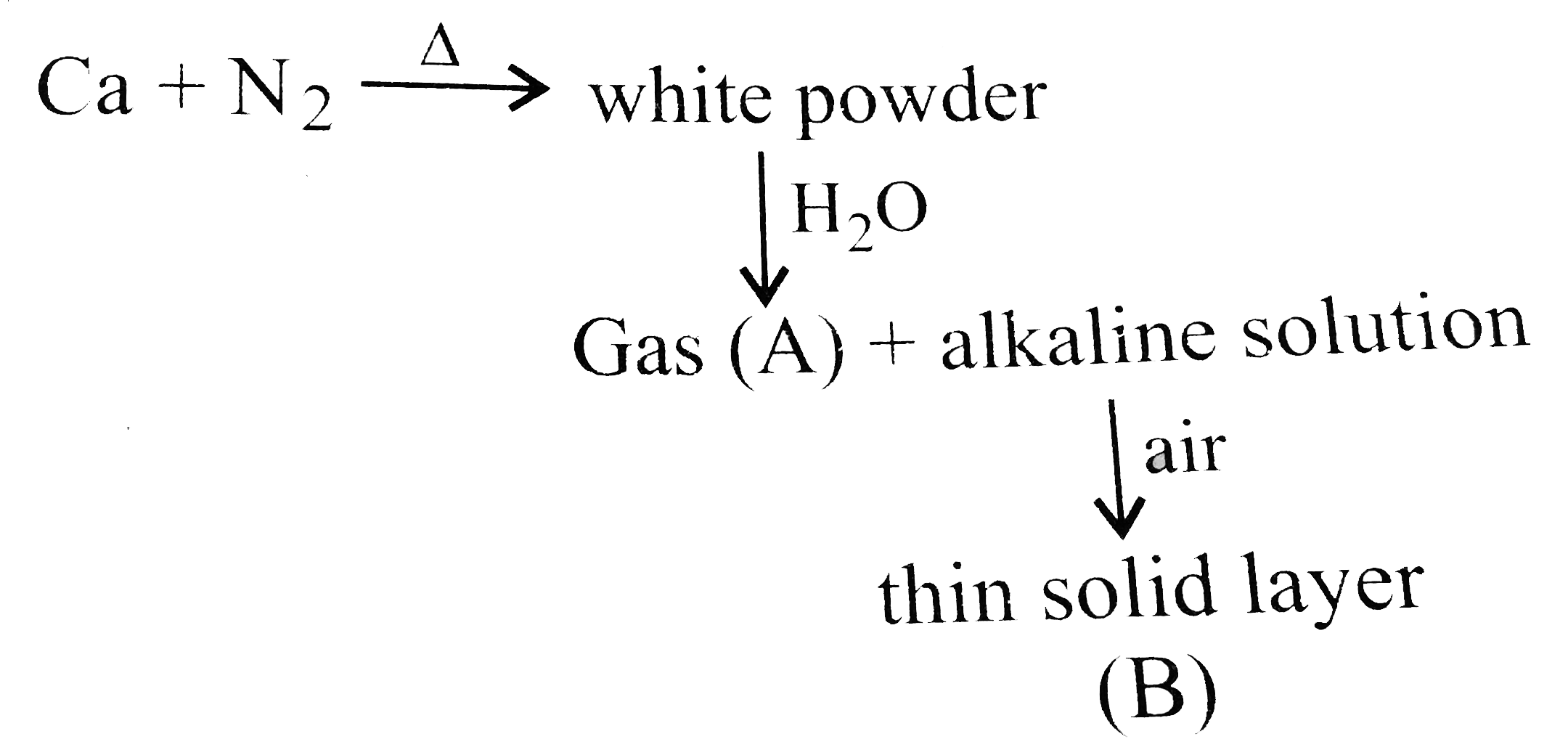Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Ex 3.1|4 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Ex 3.2|36 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|1 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives Subjective|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-HYDROGEN, WATER AND HYDROGEN PEROXIDE-Solved Examples
- A 5.0 mL of solution of H(2)O(2) liberates 0.508 g of iodine from acid...
Text Solution
|
- To a 25 mL H(2)O(2) solution excess of an acidified solution of potass...
Text Solution
|
- Element (A) burns in nitrogen to give an ionic compound, (B) reacts wi...
Text Solution
|
- Calculate the volume of 10 volume H(2)O(2) solution that will react wi...
Text Solution
|
- An aqueous compound of an inorganic compound (X) shows the following r...
Text Solution
|
- 3.4 g sample of H(2)O(2) solution containing x% H(2)O(2) by weight req...
Text Solution
|
- If 100 mL of acidified 2NH(2)O(2) is allowed to react with KMnO(4) sol...
Text Solution
|
- Calcium burns in nitrogen to produce a white powder which dissolves in...
Text Solution
|