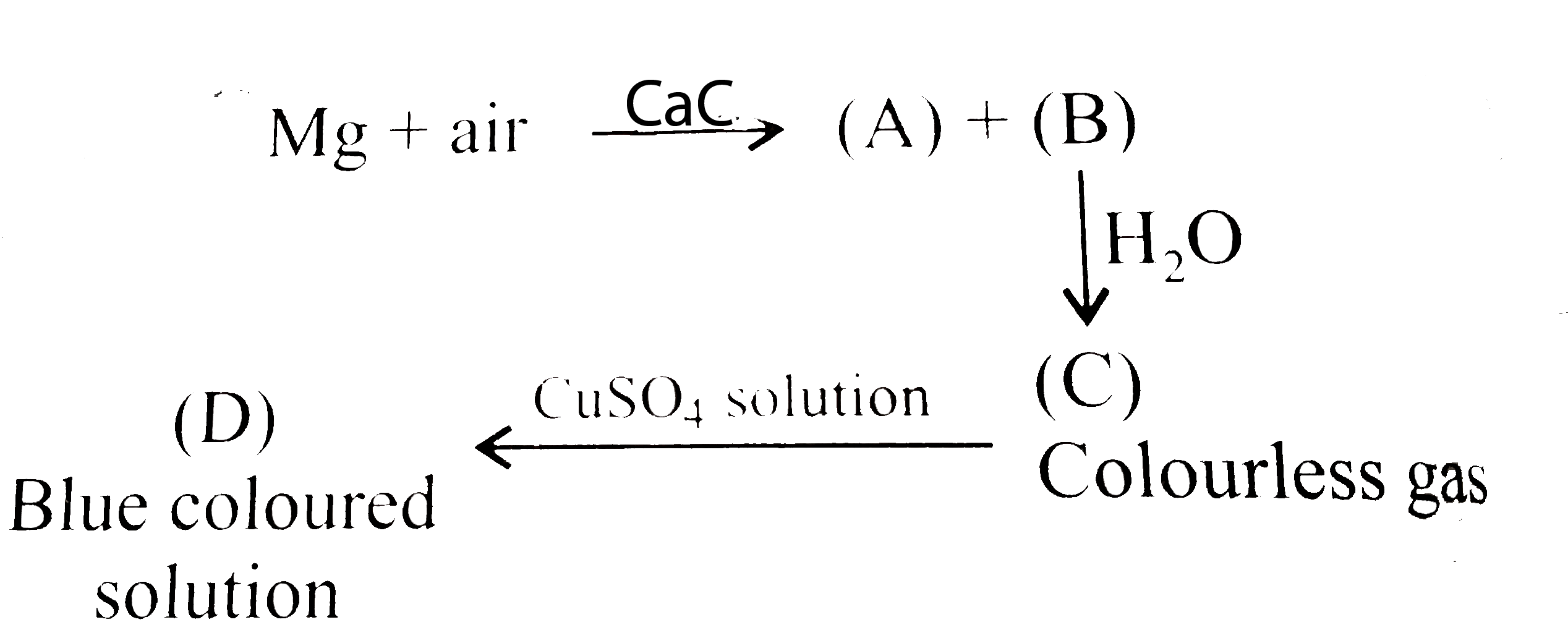Text Solution
Verified by Experts
Topper's Solved these Questions
S-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|42 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|25 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Ex 5.1 Objective|2 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Archives Subjective|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-S-BLOCK GROUP 2 - ALKALINE EARTH METALS-Solved Example
- Chemical (X) is used for water softening to remove temporary hardness....
Text Solution
|
- Magnesium on heating in air gives (A) and (B). On reaciton with water ...
Text Solution
|
- Thermal decompositon of a compound (X) yields , a basic oxide (Y) and...
Text Solution
|
- Identify (A),(B) and (C ).
Text Solution
|
- When a colourless gas (A), which is poisonous and burns with blue flam...
Text Solution
|
- An element (A) of group 2 gives brick red colour in the Bunsen flame. ...
Text Solution
|