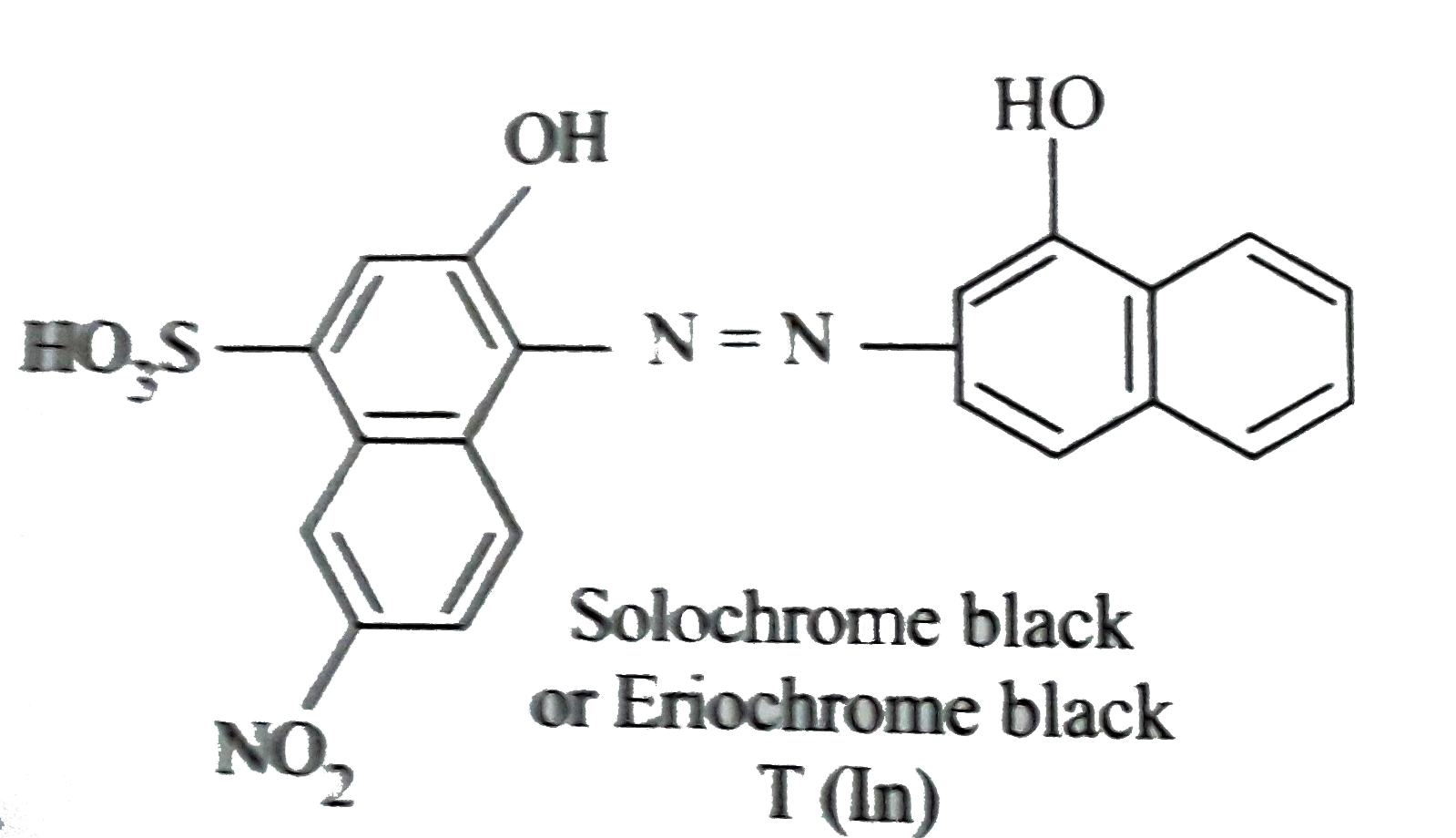
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
S-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|82 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|17 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|42 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Archives Subjective|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-S-BLOCK GROUP 2 - ALKALINE EARTH METALS-Exercises Multiple Correct
- Which of the following groups of elements have properties that are mos...
Text Solution
|
- Which of the following groups of elements have properties that are mos...
Text Solution
|
- Magnesium burns in the atmoshphere of the following gases?
Text Solution
|
- Which of the following properties show a reverse trend in moving down ...
Text Solution
|
- In which of the following, hydration enthalpy is greater than the lat...
Text Solution
|
- Which of the following statements (s) is/are not true about the diago...
Text Solution
|
- yellow phosphorus on reaction with Ca(OH)(2) gives:
Text Solution
|
- Which of the following pairs can be distinguished by the action of hea...
Text Solution
|
- The hydration entahphy of Mg^(2+) ion is higer than that of
Text Solution
|
- Which among the following has the tendency to form covalent compounds?
Text Solution
|
- Identify the correct statement(s).
Text Solution
|
- Select the correct statements about barium:
Text Solution
|
- Which of the following oxides have rock salt structure with coordinati...
Text Solution
|
- Mg and Zn have the following resemblance:
Text Solution
|
- Be and Al have the following resemblance due to diagonal relationship,
Text Solution
|
- The correct statement(s) is//are
Text Solution
|
- Which of the following metal(s) do (es) not give characteristic flame ...
Text Solution
|
- Gypsum on heating gives
Text Solution
|
- Dolotime is a mineral of
Text Solution
|
- Mg^(2+) can be detected and esitimated in hard water by titrating with...
Text Solution
|