Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Ex (Subjective)|21 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Ex (Objective)|19 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Archives )Subjective|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 14 - CARBON FAMILY-Solved Examples
- Identify(A) based on followihng facts : a. A reduces HgCl(2) solutio...
Text Solution
|
- Oixalic acid on stragheating gives (A) and (B) which are gaseous prouc...
Text Solution
|
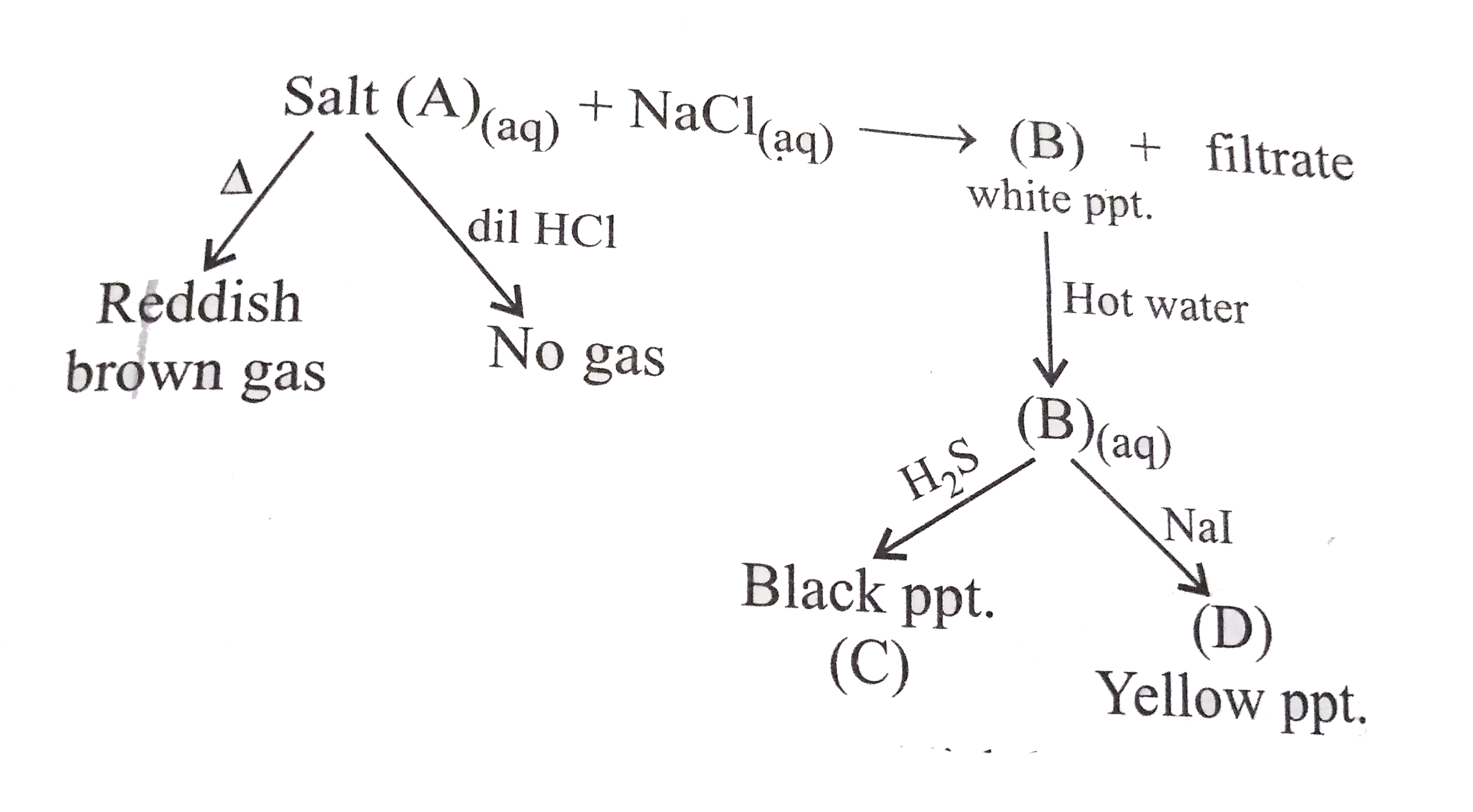
- An awueous solution of salt (A) gives a give a whes a white precipitat...
Text Solution
|
- Statring form SiCl4 prepare the following in steps not exceeding the a...
Text Solution
|
- An element of group 14 form a red coloured mized oxide (A) which on t...
Text Solution
|
- CaCO3 on beating gives a wbhite solud (A) and a gas (B), (A) on deatin...
Text Solution
|
- Chooser the correct option : a. A mixtrue of two fgasses is formed w...
Text Solution
|
- a .(NH4)4CS3 b. CaCS3 c. Crn2 , d. H2 C3 N3 O3 e. HCNS f. N...
Text Solution
|
- HgCl2 and SmCl2 cannot exist together in an aqeous solution . Explain.
Text Solution
|