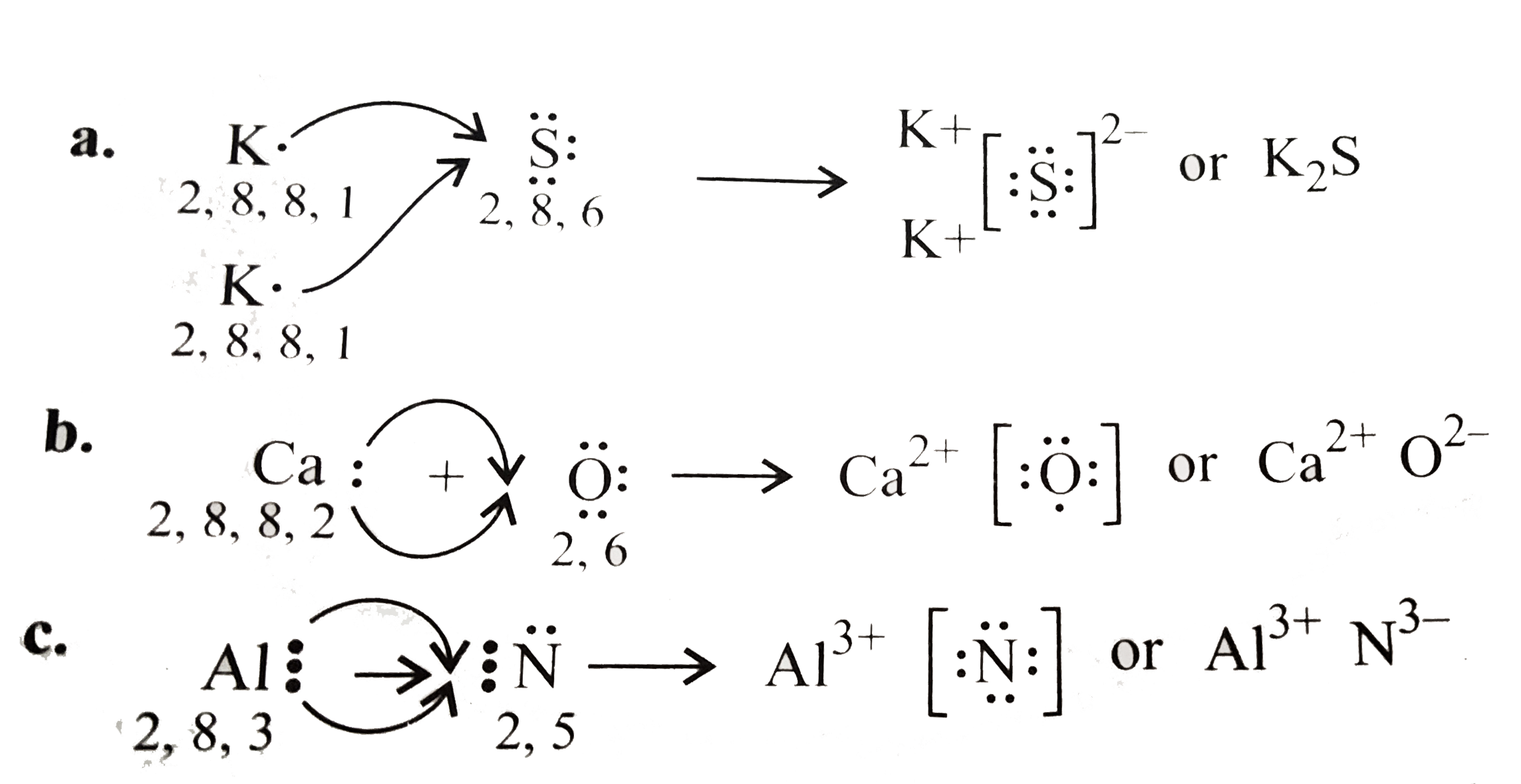Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise Exercise very short|70 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise Exercise Short|146 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 Videos
Similar Questions
Explore conceptually related problems