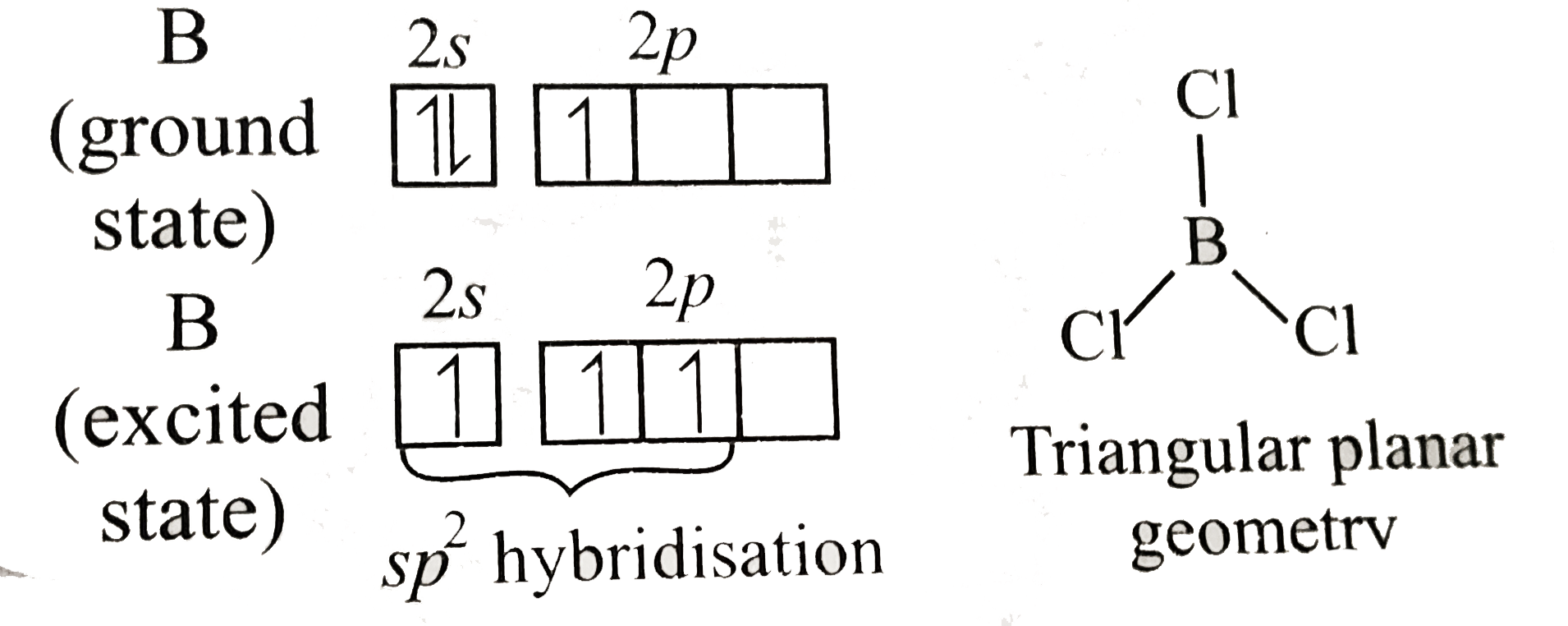
Compounds in which the central atom either does not have eight electrons in the valence shell or which have eight electrons in their valence shell but can expand their coordination number beyond four due to the presence of vacant `d`-orbitals are known as electron-deficient compounds.
In `BCl_(3)`, B has six electrons in the valence shell, thus , `BCl_(3)` behave as an electron deficiant compound.
Due to the presence of vacant `2p` orbital, `B` in `BCl_(3)` can accept a pair of electron from any donor molecule or ion and hence can form addition compound or adduct, e.g.
`Cl_(3)B+:NH_(3)to[Cl_(3)Blarr:NH_(3)]`
In `SiCl_(4)`, the central `Si` atom has eight electrons, but it can expand its coordination number beyond `4` due to the presence of vacant `3d` orbital. So `SiCl_(4)` should behave as an electron-deficient compound. However, it does not accept two more `Cl^(ө)` ions to form `[SiCl_(6)]^(2-)` because of the following two reasons:
a. Six large size `Cl^(ө)` ions cannot be accommodated around small `Si` atom.
b. Due to larger size of `Cl`, interaction between lone pair of chlorine atom and `d`-orbital of `Si`-atom will be weak.
