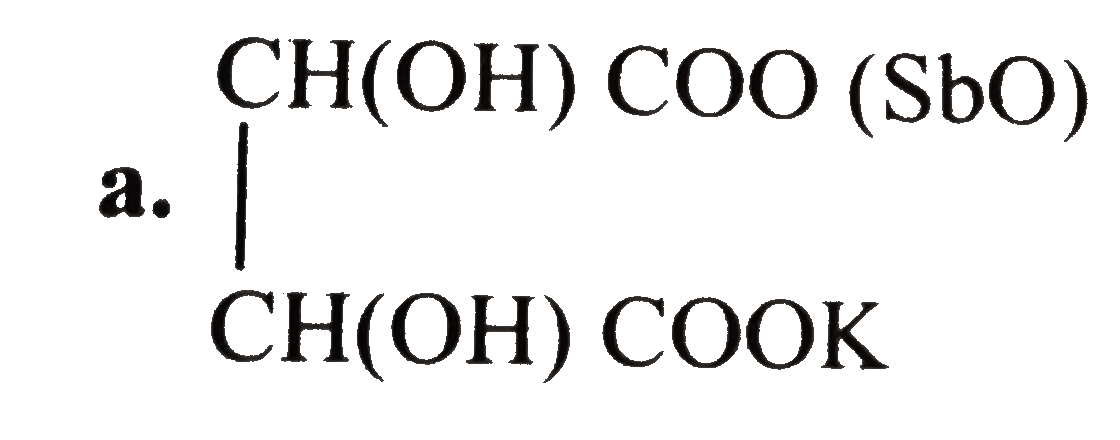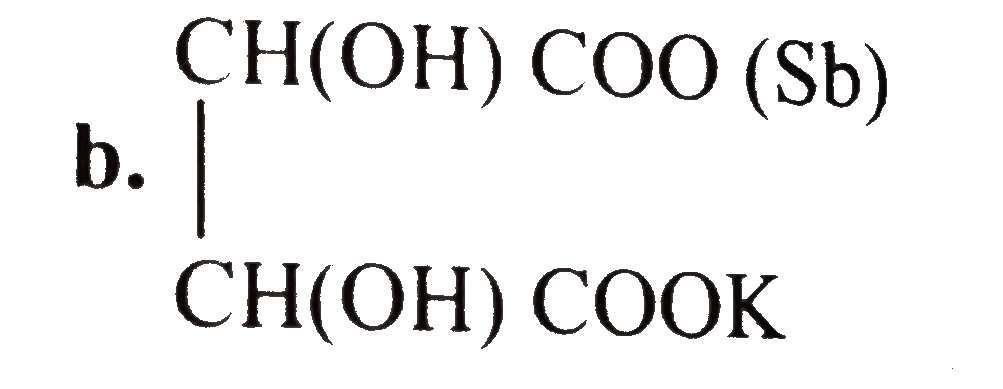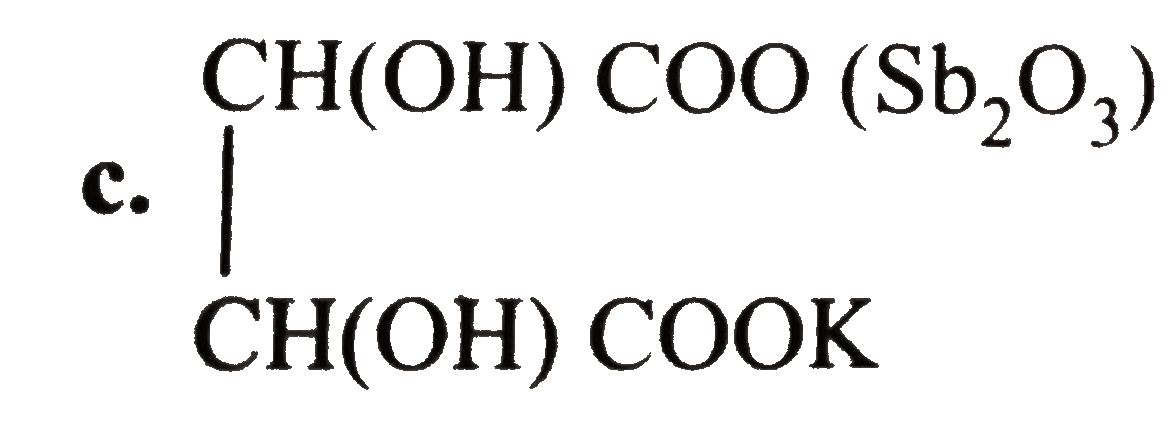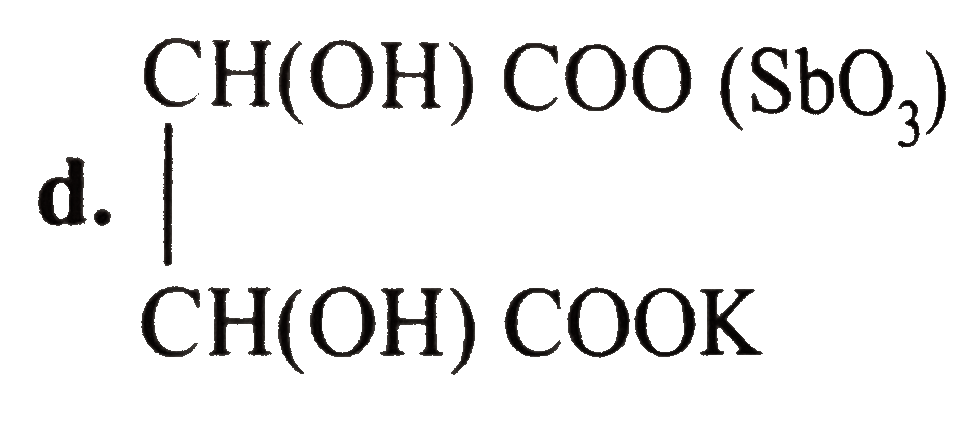A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Integer)|20 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Fill In The Blanks)|15 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|12 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Archives Analytical And Descriptive|24 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY-Exercises (Single Correct)
- Group 15 elements are commonly known as.
Text Solution
|
- Paris green was used as a pigment due to unique light green colour but...
Text Solution
|
- Tartar emetic (potassium antimony tartrate) is used as an emitic in sm...
Text Solution
|
- Which of the following halide does not hydrolyse
Text Solution
|
- Witting reagent is used for the synthesis of alkenes from ketone in or...
Text Solution
|
- Pernitric acid is
Text Solution
|
- Nitrates of all metals are
Text Solution
|
- Following tests are shown by (i) Decolourisation of acidified soln. ...
Text Solution
|
- Which of the following acids possesses oxidising, reducing, and comple...
Text Solution
|
- Acidic hydride of nitrogen is
Text Solution
|
- Among the trihalides of nitrogen, which is the least basic ?
Text Solution
|
- PCl5 exists but NCl5 does not because :
Text Solution
|
- PCl5 and PH3 exist but PH5 does not because
Text Solution
|
- The structure of phosphide ion is smaller to that of
Text Solution
|
- Calcium phosphide is used in smoke screen because it.
Text Solution
|
- Holme's signals produce burning gases which serve as a signal to the a...
Text Solution
|
- Among the following the strongest acid is
Text Solution
|
- Basicity of H3 PO4 and H3 PO3 are
Text Solution
|
- Phosphoric acid is syrupy in nature due to hydrogen bonding. Each mole...
Text Solution
|
- PCl3, P4 O6 and P4 O10, PCl5 on hydrolysis gives respectivey.
Text Solution
|