Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (True/False)|2 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Archives Analytical And Descriptive|24 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY-Exercises Archives (Subjective)
- Give balanced equations for the following : Phosphorous reacts with ...
Text Solution
|
- Write the balanced chemical equations when hypophosphorus acid is heat...
Text Solution
|
- Explain the following (i)H3 PO3 is a dibasic acid. (ii) Phosphine ...
Text Solution
|
- Write balanced equation for (i) The preparation of phosphine from Ca...
Text Solution
|
- Write two resonance structure of N2 O that satisfy the octet rule.
Text Solution
|
- Write balanced chemical equations for the following : (i) Sodium ni...
Text Solution
|
- Arrange the following as stated : Increasing order of the extent of ...
Text Solution
|
- Give reason in one or two sentences. "Ammonium chloride is acidic in...
Text Solution
|
- Complete and balance the following chemical reactions : Red phosphorus...
Text Solution
|
- Identify the compounds A and B. PCl5 + SO2 A + B rarr.
Text Solution
|
- Complete and balance the following reactions. Ca5(PO4)3 F + H2 SO4 +...
Text Solution
|
- Account for the following : (i)The experimentally determined N-F bon...
Text Solution
|
- Draw the structure of P4 O10 and identify the number of single and dou...
Text Solution
|
- A soluble compound of a poisonous element M, when heated with Zn//H2 S...
Text Solution
|
- Reaction of phosphoric acid with Ca5(PO4)3 F yields a fertiliser 'trip...
Text Solution
|
- Complete and balance the following equation : P4 O10 + PCl5 rarr.
Text Solution
|
- In the following equation : A + 2 B + H2 O rarr C + 2 D A = HNO2, ...
Text Solution
|
- Give reason, why elemental nitrogen exists as a diatomic molecule, whe...
Text Solution
|
- Write the balanced equations for the reactions of the following compou...
Text Solution
|
- How many grams of CaO are required to neutralise 852 g of P4 O10 ? Dra...
Text Solution
|
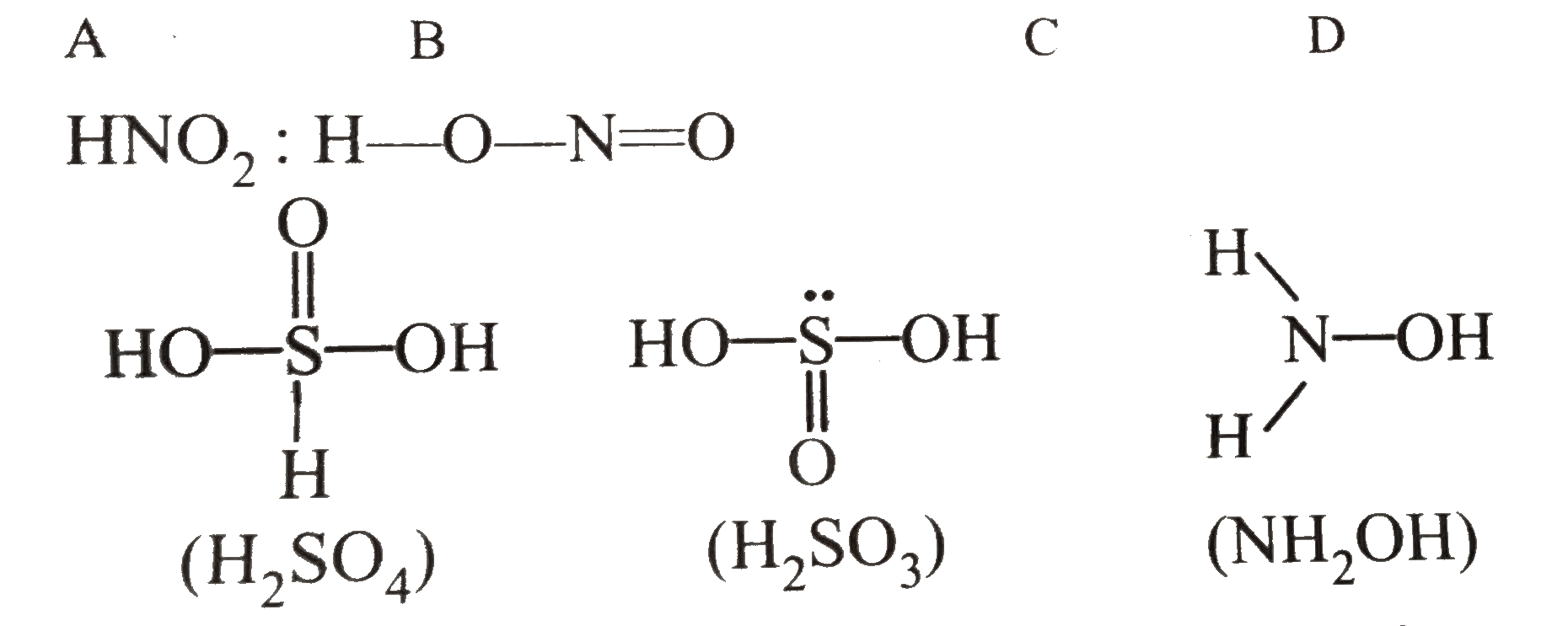 .
.