A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Integer|4 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives (Fill In The Blanks)|1 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Multiple Correct|1 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|28 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise True/False (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY-Archives Single Correct
- The oxide that gives H(2)O(2) on treatment with a dilute acid is
Text Solution
|
- There is no S-S bond in
Text Solution
|
- The oxidation states of the most electronegative element in the produc...
Text Solution
|
- The species that does not contain peroxide bond is //are :
Text Solution
|
- Hydrolysis of one mole of peroxodisulphuric acid produces
Text Solution
|
- Which one of the following oxides is neutral?
Text Solution
|
- Amongst H2O, H2S, H2 Se and H2 Te, the one with the highest boiling po...
Text Solution
|
- The number is S - S bonds in sulphar trioxide times S(3)O(9) is
Text Solution
|
- Which of the following will not be oxidized by O3 ?
Text Solution
|
- The species having pyramidal shape is
Text Solution
|
- Which of the following does not give oxygen on heating ?
Text Solution
|
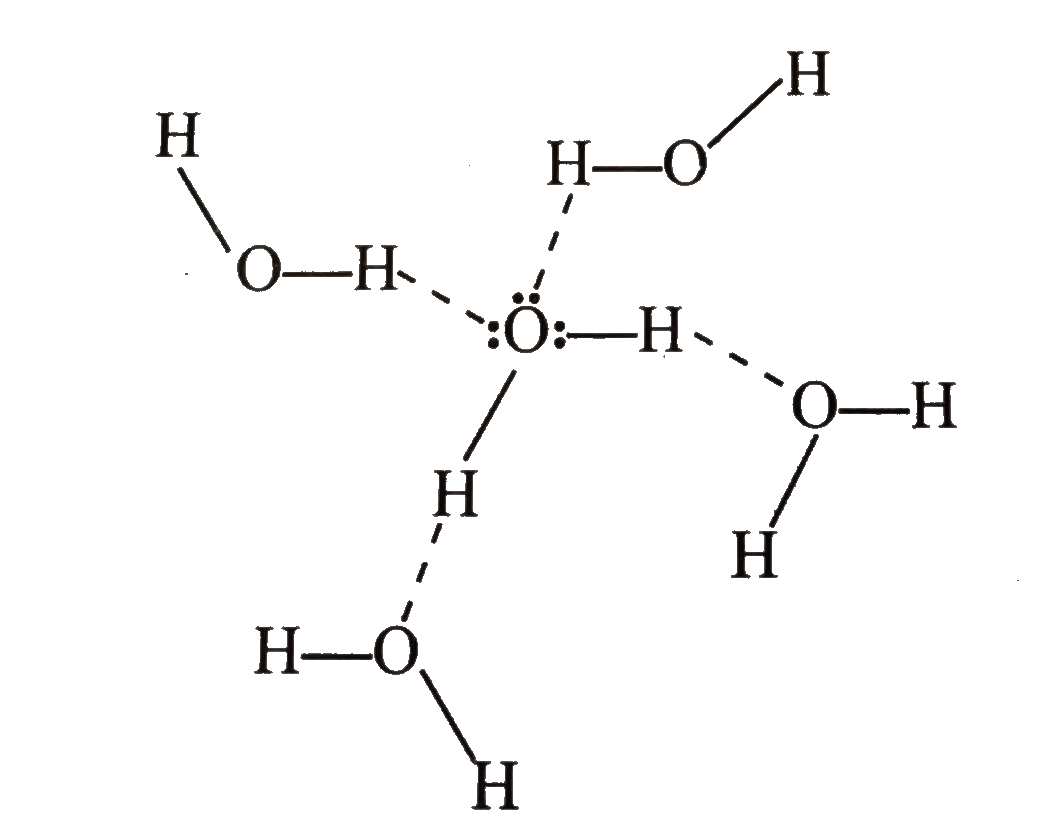 .
.