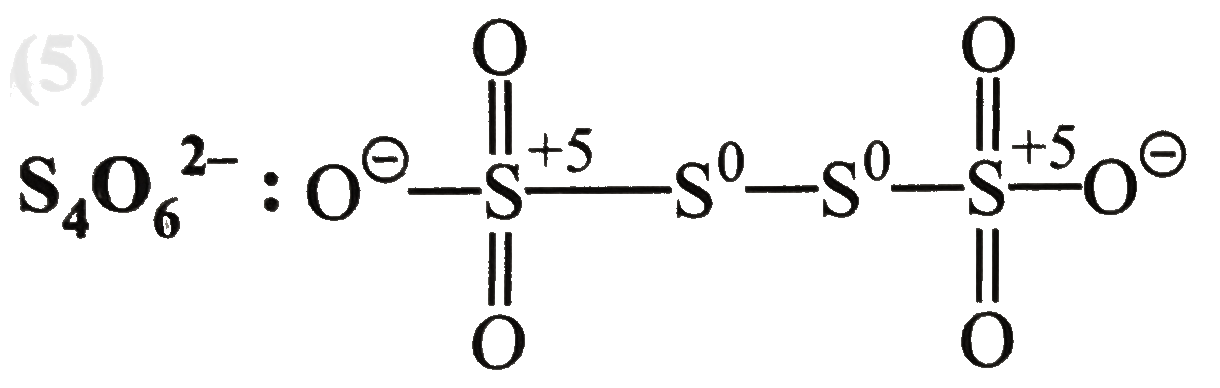Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives (Fill In The Blanks)|1 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Single Correct|11 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|28 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise True/False (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY-Archives Integer
- Among the following , the number of elements showing only one non-zero...
Text Solution
|
- The value of n in the molecular fromula Ben Al2Si6 O(18).
Text Solution
|
- The difference in the oxidation numbers of two types of sulphul atoms ...
Text Solution
|
- A list of species having the formula XZ(4) is given below XeF(4),SF(4)...
Text Solution
|