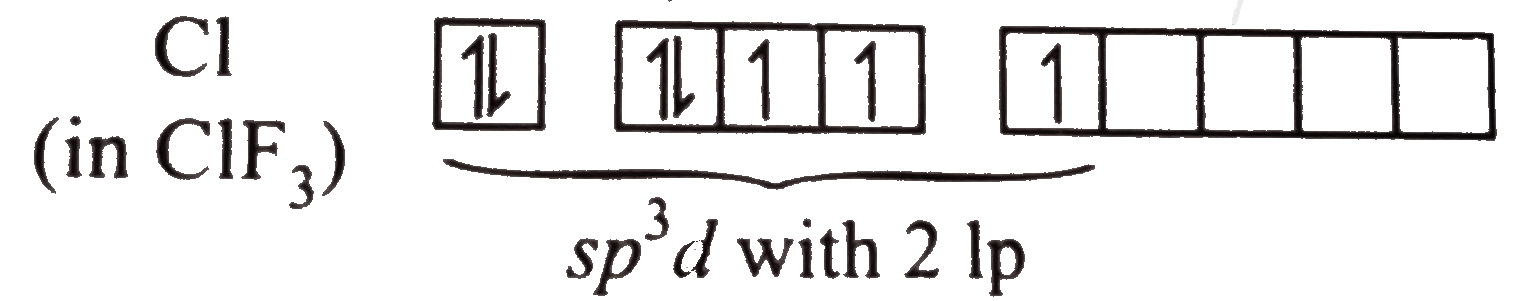A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|14 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|70 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exs 4.1 (Objective)|7 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-Exercises (Linked Comprehension)
- Halogens react with each other to form a number of compounds called in...
Text Solution
|
- Halogens react with each other to form a number of compounds called in...
Text Solution
|
- Halogens react with each other to form a number of compounds called in...
Text Solution
|
- Halogens react with each other to form a number of compounds called in...
Text Solution
|
- I(2) has less solubility in water and its solubility increase on addin...
Text Solution
|
- I(2) has less solubility in water and its solubility increase on addin...
Text Solution
|
- I(2) has less solubility in water and its solubility increase on addin...
Text Solution
|
- I(2) has less solubility in water and its solubility increase on addin...
Text Solution
|
- Oxygen is more electronegative than chloride. In the series of oxyacid...
Text Solution
|
- Oxygen is more electronegative than chloride. In the series of oxyacid...
Text Solution
|
- Oxygen is more electronegative than chloride. In the series of oxyacid...
Text Solution
|
- Oxygen is more electronegative than chloride. In the series of oxyacid...
Text Solution
|
- Among the halogens, fluorine differs considerably form the other membe...
Text Solution
|
- Among the halogens, fluorine differs considerably form the other membe...
Text Solution
|
- Among the halogens, fluorine differs considerably form the other membe...
Text Solution
|
- Bleaching power is a mixed salt of hydrochloric acid and hypochlorious...
Text Solution
|
- Bleaching power is a mixed salt of hydrochloric acid and hypochlorious...
Text Solution
|
- Bleaching power is a mixed salt of hydrochloric acid and hypochlorious...
Text Solution
|
- Bleaching power is a mixed salt of hydrochloric acid and hypochlorious...
Text Solution
|
- Bleaching power is a mixed salt of hydrochloric acid and hypochlorious...
Text Solution
|