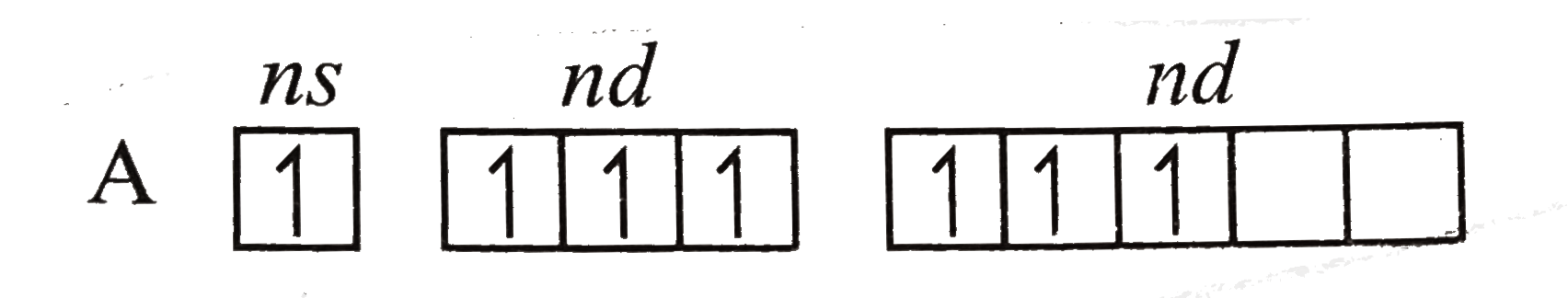Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Fill In The Blanks)|15 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (True/False)|14 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Assertion Reasoning)|13 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-Exercises (Integer)
- Sodium iodate is treated with calculated amount of sodium bisulphite t...
Text Solution
|
- In the molecule ICI(3), how many lone pairs of electrond are associate...
Text Solution
|
- In the interhalogen compound AB(n), what is the maximum value of n ?
Text Solution
|
- In a given sample of bleaching powder, the precentage of avilable chlo...
Text Solution
|
- What is the oxidation state of iodine in H(5)IO(6) ?
Text Solution
|
- How many orbitals are involved in the hybridisation of idione in IF(7)...
Text Solution
|
- Chlorine water on cooling deposits greenish yellow crystals of formula...
Text Solution
|
- How many lone pairs are associted with in IF(7) ?
Text Solution
|
- What is the oxidation state of CI in HCIO(4) ?
Text Solution
|
- What is the oxidation state of CI in HCIO(4) ?
Text Solution
|