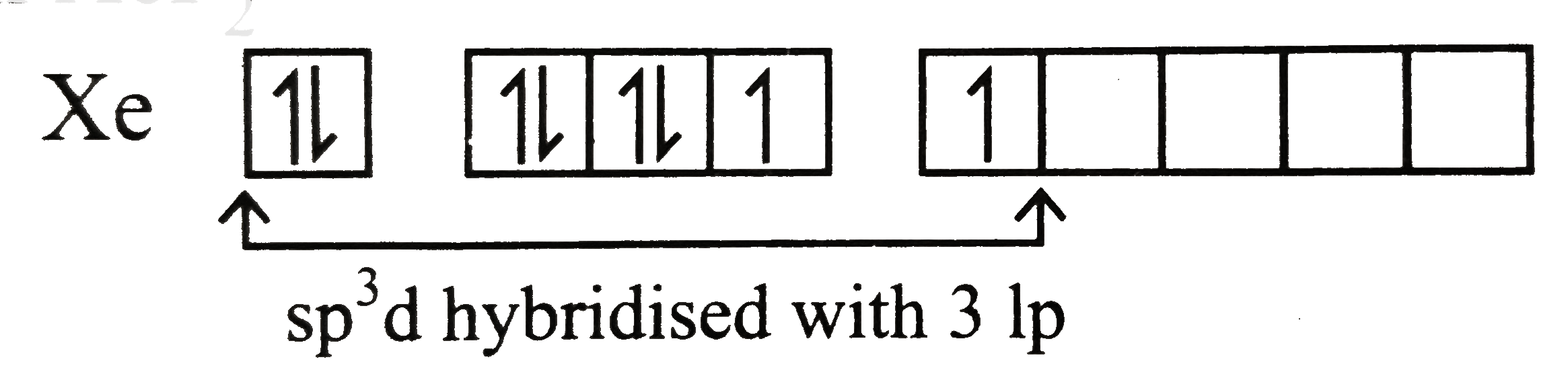Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (Fill In The Blanks)|15 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (True And False)|11 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (Assertion And Reason)|12 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise True/False (Subjective)|14 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 18 ELEMENTS - THE INERT GASES-Exercises (Integer)
- What is the oxidation number of Xe in XeOF(2)?
Text Solution
|
- What is the total number of electron present in the last orbit of argo...
Text Solution
|
- What is the percentage of argon in air ?
Text Solution
|
- What is the total number of unpaired eletrons in inert gas ?
Text Solution
|
- What is the total number of lons pair of electron present in Xe in XeF...
Text Solution
|
- What is the oxidation sate of XeF(6)?
Text Solution
|
- How many dpi-per bonds are there in XeO(4)?
Text Solution
|