Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Ex 6.2 Subjective (Compound Of Cu:)|3 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Ex 6.2 Subjective (Compound Of Zn:)|1 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Ex 6.1 Subjective|12 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Subjective|35 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-D AND F BLOCK ELEMENTS-Ex 6.2 Subjective (Compound Of Fe :)
- Complete and balance the following equation: (i). FeSO4underset(2.K4...
Text Solution
|
- Explain the following : (i). FeCl3 and FeBr3 are well known, but FeI...
Text Solution
|
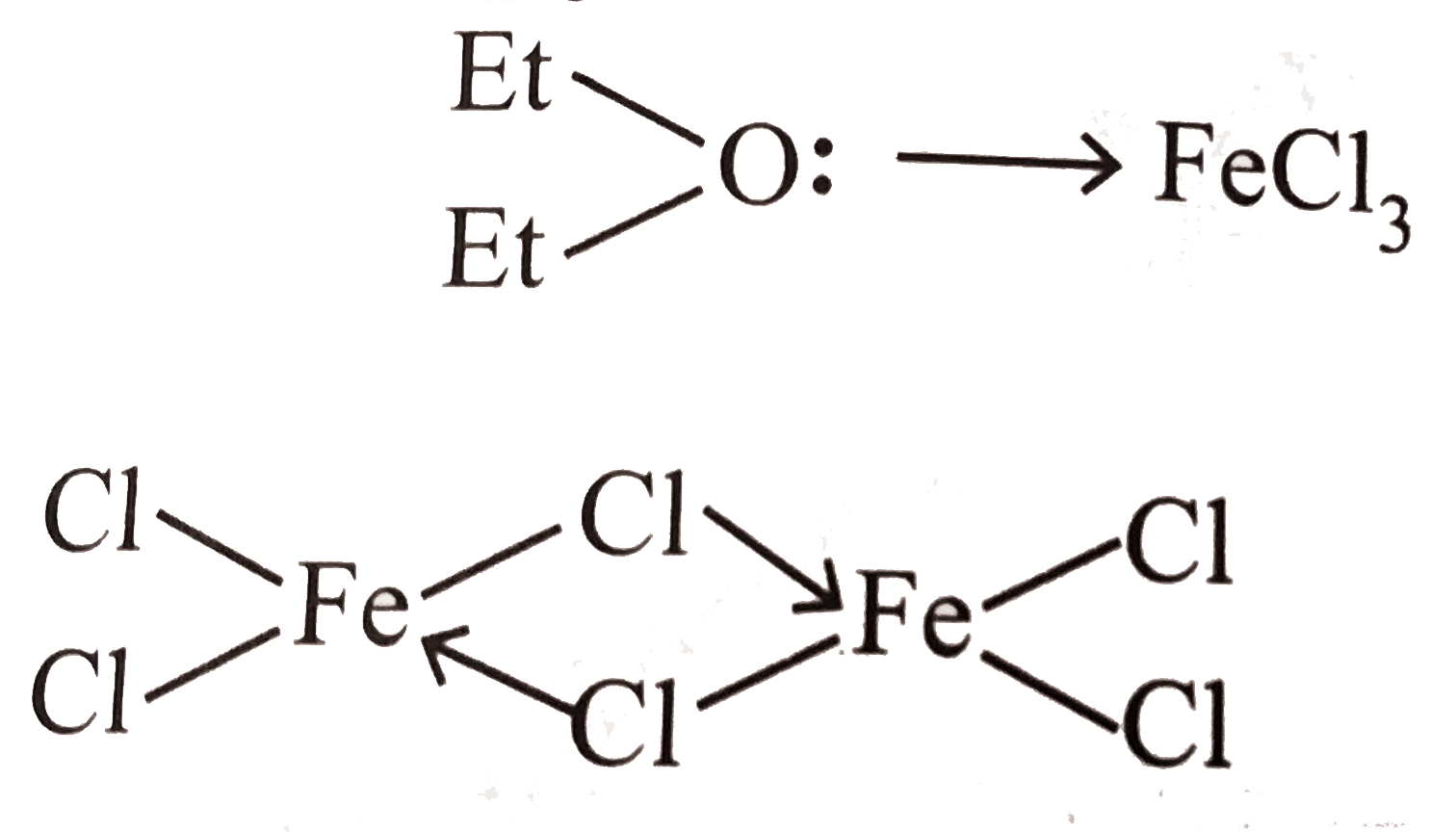
- Draw shape of FeCl3 in : (i). Water (ii). Ether (iii). Gaseous s...
Text Solution
|
- What happen when iron is treated with: (i). Steam (ii). Dilute HNO...
Text Solution
|
- Colourless salt (A) decolourise I(2) solution and gives white precipit...
Text Solution
|
- Iron forms iron (II) chloride, FeCl2 and iron (III) chloride FeCl3. On...
Text Solution
|
- Account for the following observation and write balanced chemical equa...
Text Solution
|