Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Exercises Fill In The Blanks|10 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Exercises True False|10 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|20 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Subjective|35 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-D AND F BLOCK ELEMENTS-Exercises Integer
- What is the value of x in the following equation. Cr2O7^(2-)+8H^(o+)...
Text Solution
|
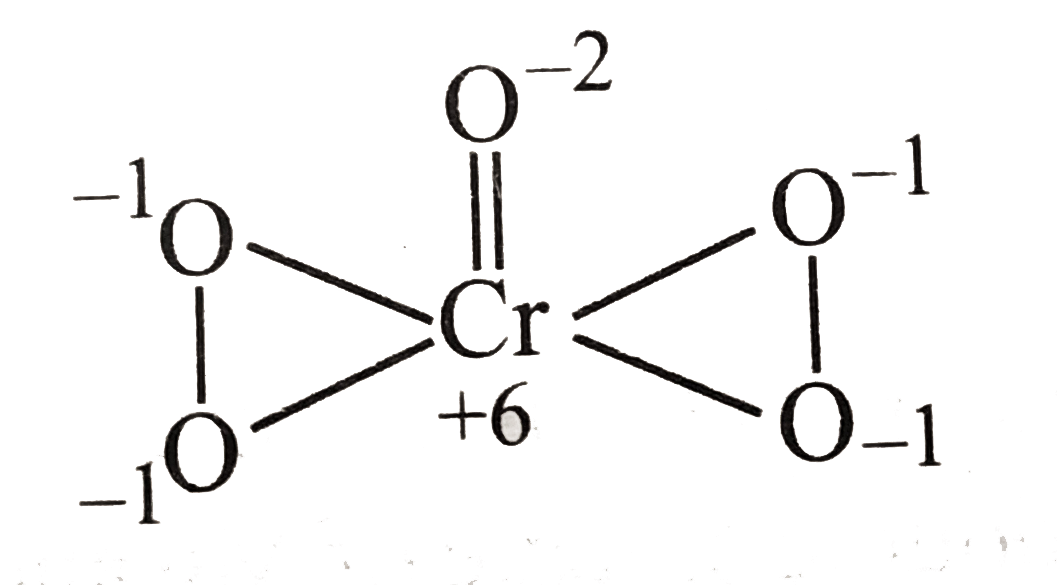
- What is the oxidation states of Cr in butterfly struncture.
Text Solution
|
- What is the value of x in the following equation: 2MnO4^(ɵ)+3Mn^(2+)...
Text Solution
|
- Out of the following how many oxides are acidic. MnO,Mn2O3,MnO2,MnO3...
Text Solution
|
- Out of the following how many of them have magnetic moment value sqrt...
Text Solution
|
- Out of the following how many of them are coloured compounds. MnO4^(...
Text Solution
|
- Out of the following how many oxides are basic: TiO,Sc2O3,Ti2O3,VO,V...
Text Solution
|
- What is the value of x in the Wilkinson's catalyst in the hydrogenatio...
Text Solution
|
- How many of the transition elements are called coinage metals.
Text Solution
|
- How many of the transition elements are called Platinum metals.
Text Solution
|