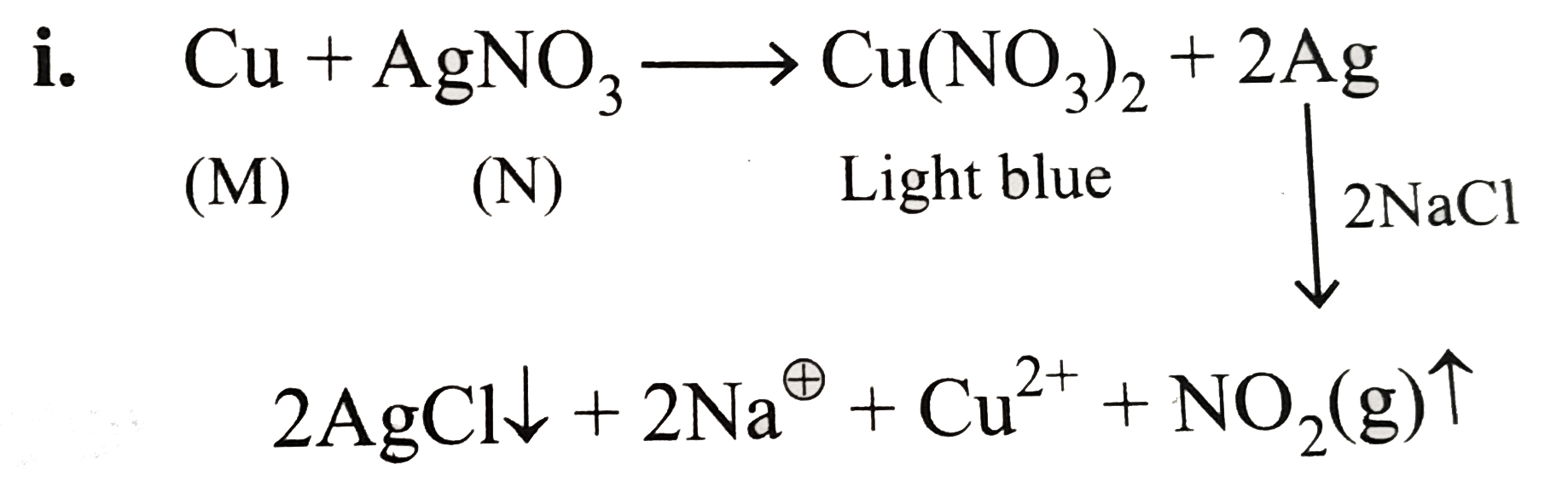
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Multiple Correct|8 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Single Correct|29 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Exercises True False|10 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Subjective|35 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-D AND F BLOCK ELEMENTS-Archives Linked Comprehension
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- Copper is the most noble of the first row transition elements it occur...
Text Solution
|
- Copper is the most noble of the first row transition elements it occur...
Text Solution
|
- In self-reduction, the reducing species is
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|