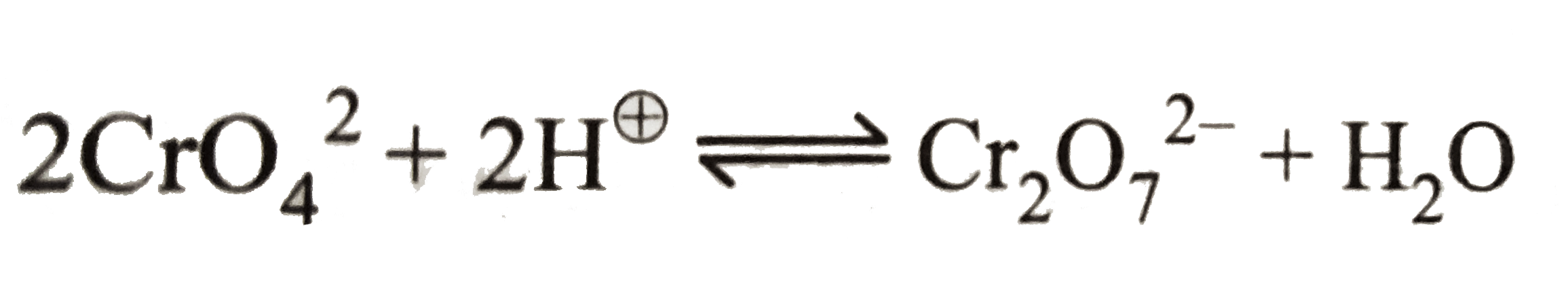
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-D AND F BLOCK ELEMENTS-Archives Subjective
- Complete and balance the following reactions: (i). Zn+NO3^(ɵ)toZn^(2...
Text Solution
|
- State the conditions under which the following preparations are carrie...
Text Solution
|
- Show with balanced equations what happens when the following are mixed...
Text Solution
|
- Write the balanced equations for the reactions when (i). Potassium p...
Text Solution
|
- Mention the products formed in the following (i) Zinc oxide is treat...
Text Solution
|
- Write the balanced equations for the reaction occuring when gold is di...
Text Solution
|
- Write the balanced equation for the following "potassium permanganate ...
Text Solution
|
- Complete and balance the following reactions: (i). Mn^(2+)+PbO2toMnO...
Text Solution
|
- Answer the following questions briefly: (i). What is the actual redu...
Text Solution
|
- Write the balanced equations for extraction of silver from glance by c...
Text Solution
|
- Write the balanced chemical equation for the following: (i). Silver ...
Text Solution
|
- Write balanced equations for "the extraction of copper from pyrites by...
Text Solution
|
- Write the balanced chemical equations for the following reactions: (...
Text Solution
|
- A light bluish-green crystalline compound responds to the following te...
Text Solution
|
- Complete and balace the following chemical reaction: (i). Na2CO3 is ...
Text Solution
|
- Compelete and balance the following: (NH4)2S2O8+H2O+MnSO4to
Text Solution
|
- The composition of a sample of wustite is Fe(0.93)O(1.00) What percent...
Text Solution
|
- Complete and balance the following reaction: (i). [MnO4]^(2-)+H^(o+)...
Text Solution
|
- Write a balanced equation for the reaction of agentite with KCN and na...
Text Solution
|
- Write a balancfed equation for the following: "Reaction of zinc with...
Text Solution
|