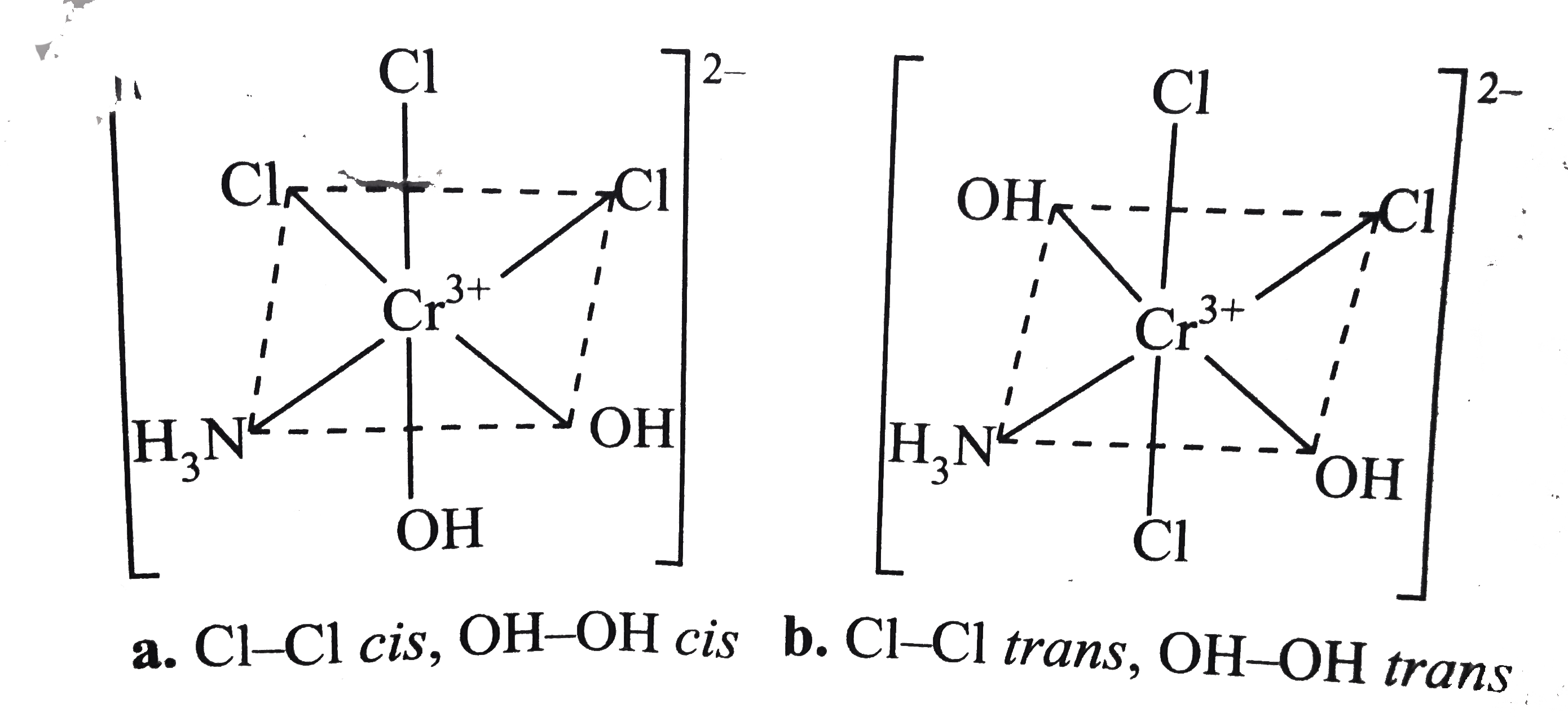Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Solved Examples|42 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Subjective (Terminology)|5 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Archives Subjective
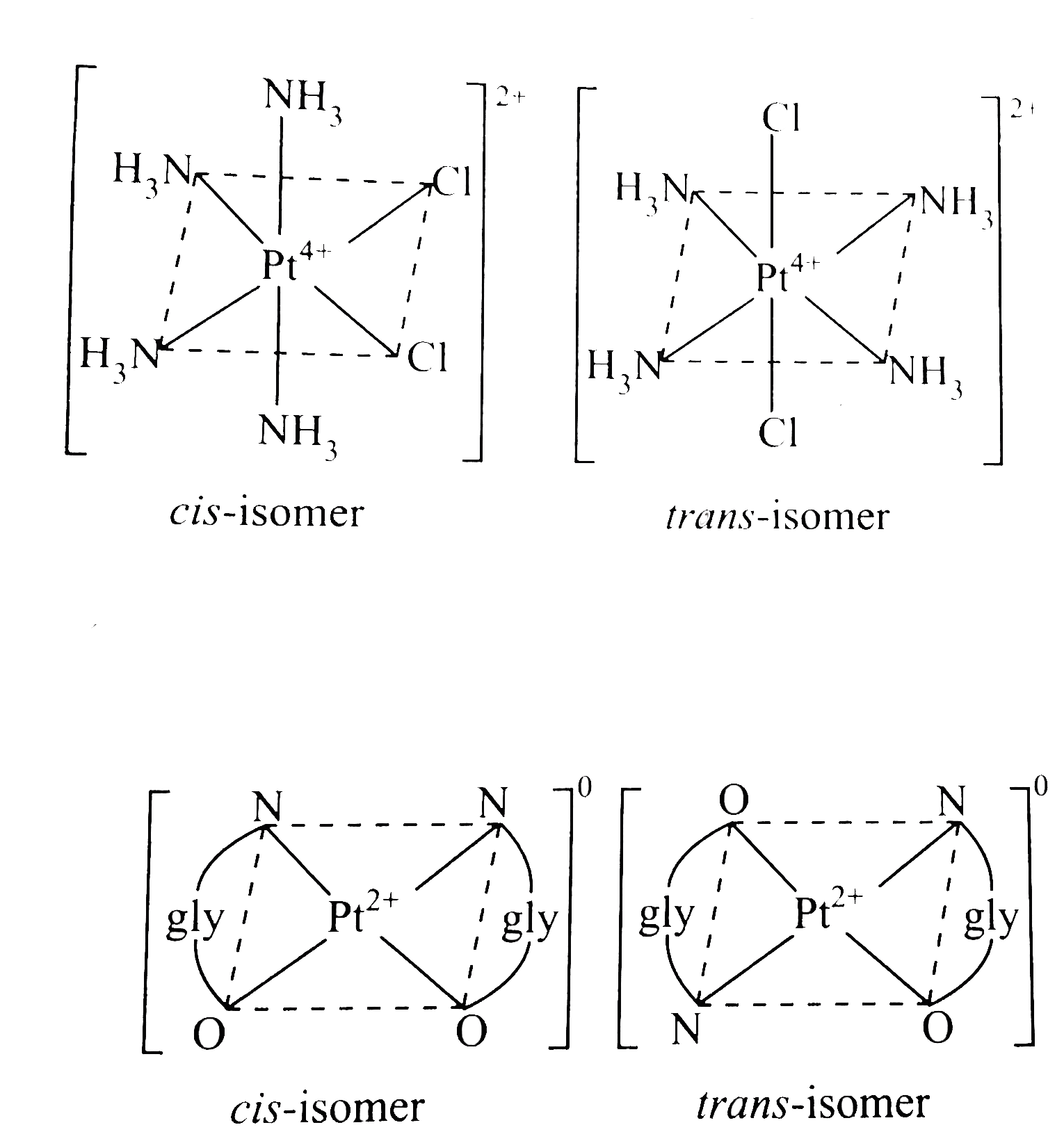
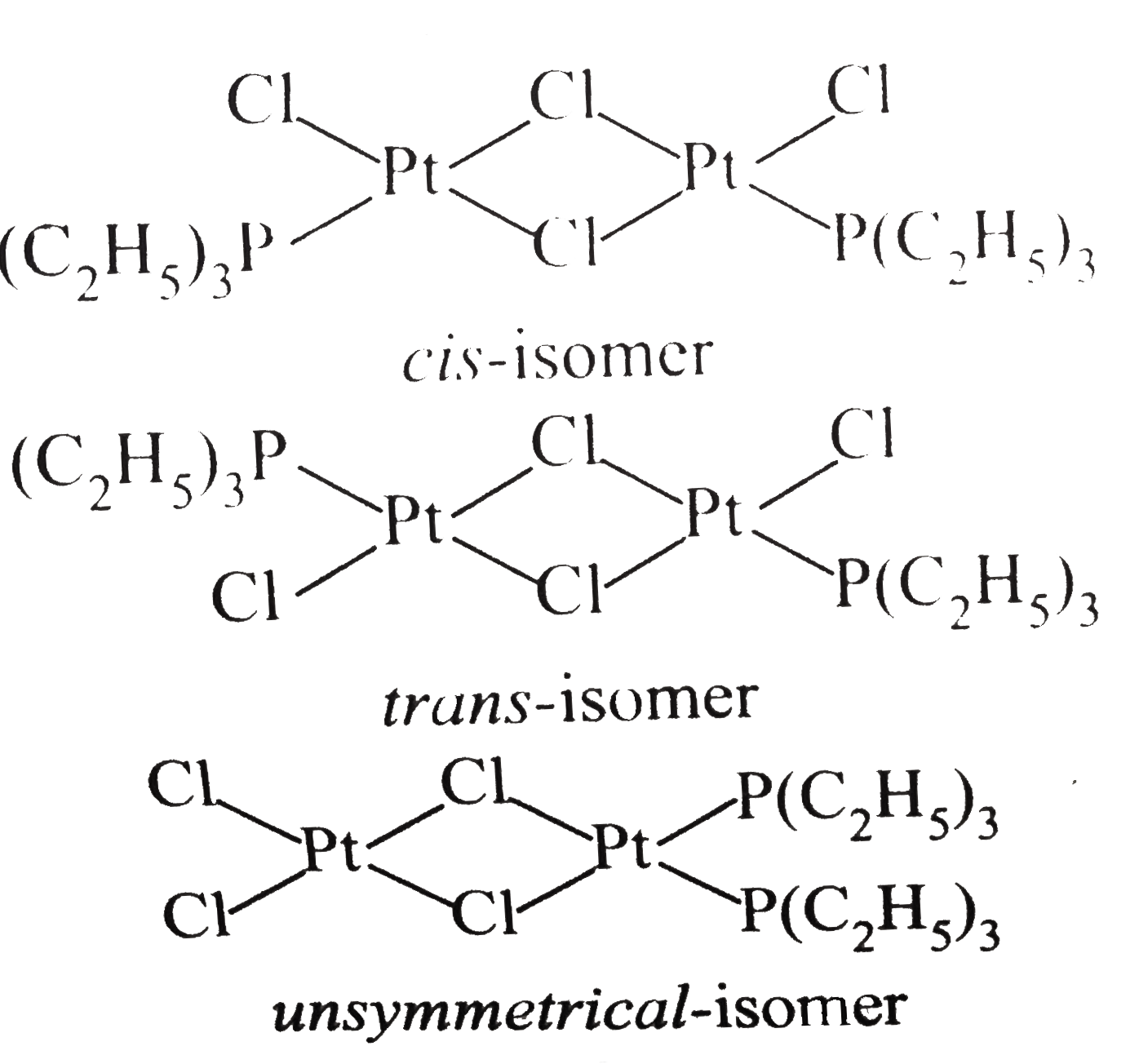
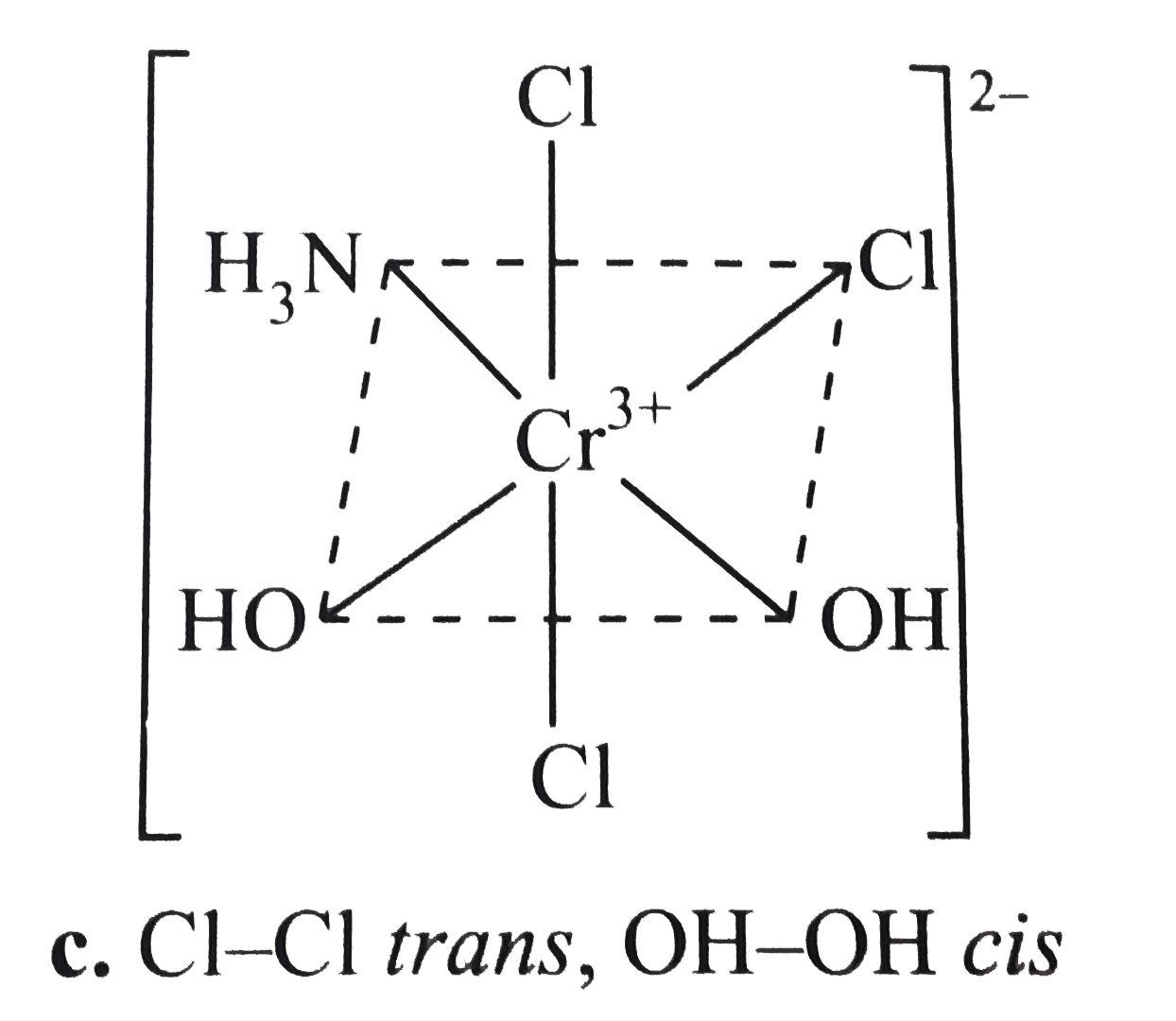
- Draw all possible isomers of (i) [Pt(NH(3))(4)CI(2)]^(2+) (ii) [Pt...
Text Solution
|
- Write the balanced chemical equations for the following "Potassium fer...
Text Solution
|
- Give reasons in two or three sentences only for the following "The s...
Text Solution
|
- The acidic aqueous solution of ferrous ion forms a brown complex in th...
Text Solution
|
- Identify the complex which are expected to be coloured Explain (a) [...
Text Solution
|
- Write the IUPAC name for the following compounds (a) [Co(NH(3))(5)O...
Text Solution
|
- Write the IUPAC name for [Cr(NH(3))(5)CO(3)]CI .
Text Solution
|
- Write a balanced equation for the reaction of argentite with KCN and n...
Text Solution
|
- Write the formulae of the following complexes (a) Pentamminechloroco...
Text Solution
|
- A,B and C are three complexes of chromium(III) with the empirical form...
Text Solution
|
- An aqueous solution containing 1 mol of HgI(2) and 2mol of Nal is ora...
Text Solution
|
- Draw the structures of [Co(NH(3))(6)]^(3+),[Ni(CN)(4)]^(2-) and [Ni(CO...
Text Solution
|
- A metal complex having composition Cr(NH(3))(4)CI(2) Br has been isola...
Text Solution
|
- Dedue the structures of [NiCI(4)]^(2-) and [Ni(CN)(4)]^(2-) considerin...
Text Solution
|
- Write the IUPAC nomenclature of the given complex along with its hybri...
Text Solution
|
- NiCI(2) in the presence of dimethy1 glyoxime (DMG) gives a complex whi...
Text Solution
|
- AIF(3) is insoluble in anhydrous HF but when little KF is added to the...
Text Solution
|
- Write the balanced chemical equations for developing a black and white...
Text Solution
|
- Fe^(3+)overset(SNC^(Θ)("Excess"))rarrunderset("Bloodre"d)(A)overset(F^...
Text Solution
|