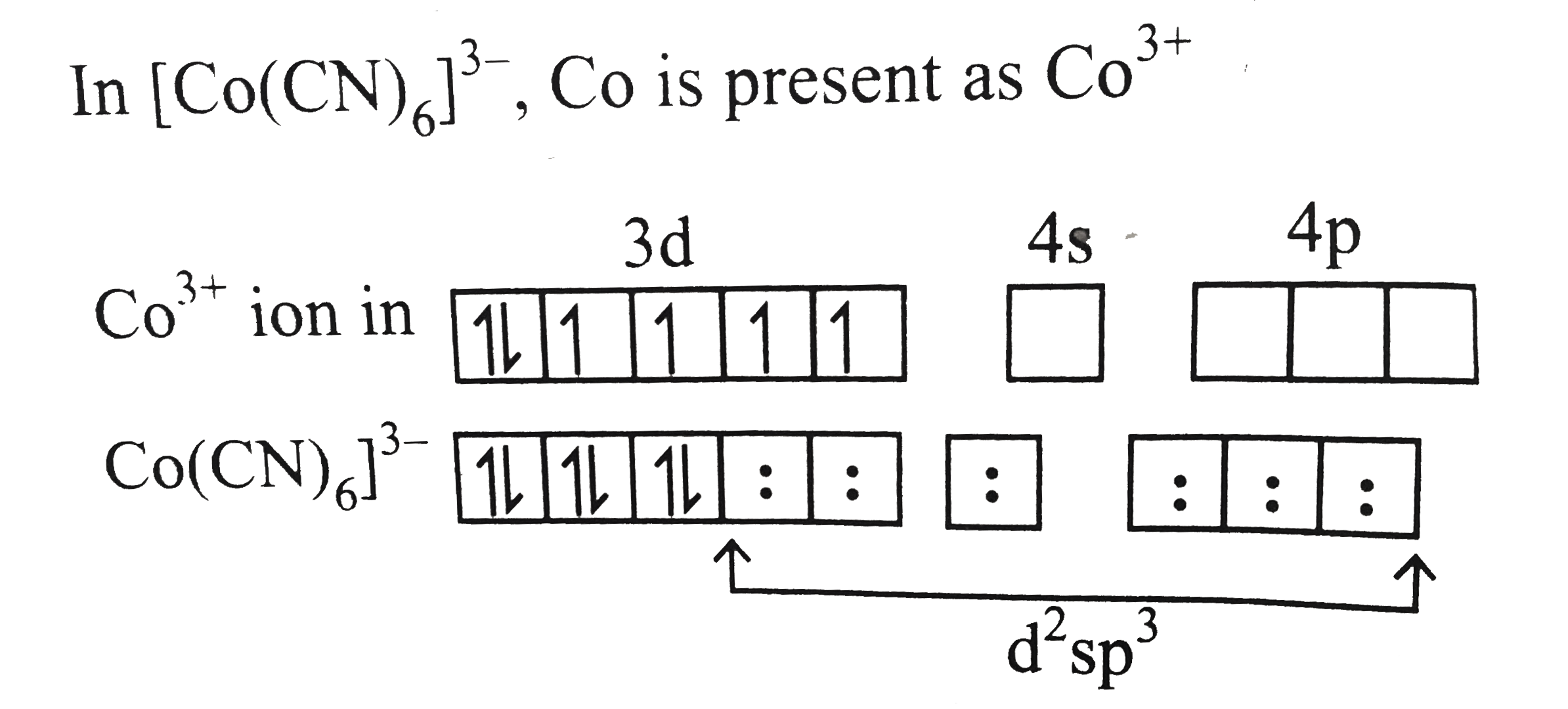
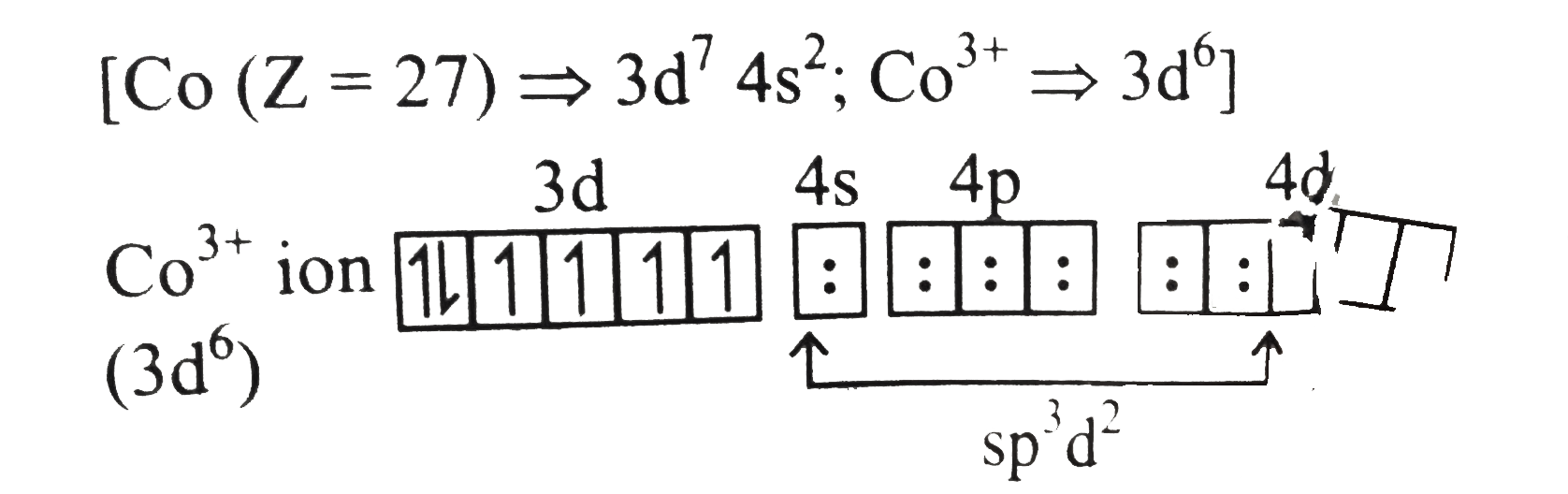Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Subjective (Terminology)|5 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Subjective (Effective Atomic Number (Ean))|3 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Solved Examples
- The formula Co(NH(3))(4) CO(3) Br represents three isomers (i) Draw ...
Text Solution
|
- On the basic VBT answer the following questions for the 4-coordinated ...
Text Solution
|
- Explain why a knowledge of magnetic suceptilbility of a complex is oft...
Text Solution
|
- Magetic moment of [CoI(4)]^(2-) is 3.8BM Using valence bond approach p...
Text Solution
|
- A complex of a certain metal ion has a magnetic moment of 4.90BM Anoth...
Text Solution
|
- Find out the number of unpaired electrons in strong and weak octahedra...
Text Solution
|
- Distinguish between the possibillities in complex ions of Delta =0 and...
Text Solution
|
- Determine the crystal field stabilisation energy of a d^(6) complex ha...
Text Solution
|
- Give reason for the fact that amongst Ni(CO)(4)[Ni(CN)(4)]^(2-) and Ni...
Text Solution
|
- One the basic of CFT predict the geometry of the compund K(3)[Mn(CN)(6...
Text Solution
|
- The enthalpy of hydration of the Fe^(2+) ion is 11.4kcal//mol higher t...
Text Solution
|
- If a complexing metal of the first transition series has a di configur...
Text Solution
|
- In terms of CFT explain why a d^(9) octahedral complex with six identi...
Text Solution
|
- Derive the geometry of the complex compound corresponding to the brown...
Text Solution
|
- Which of the electronic configuration according to crystal field theor...
Text Solution
|
- Which of the electronic configuration according to crystal field theor...
Text Solution
|
- According to crystal field therory the electronic configuration of the...
Text Solution
|
- According to crystal field theory the electronic configuration of the ...
Text Solution
|
- According to crystal field theory the electronic configuration of the ...
Text Solution
|
- Corystal field splitting energy (CFSE) for the complex [Cr(H(2)O)(6)]^...
Text Solution
|