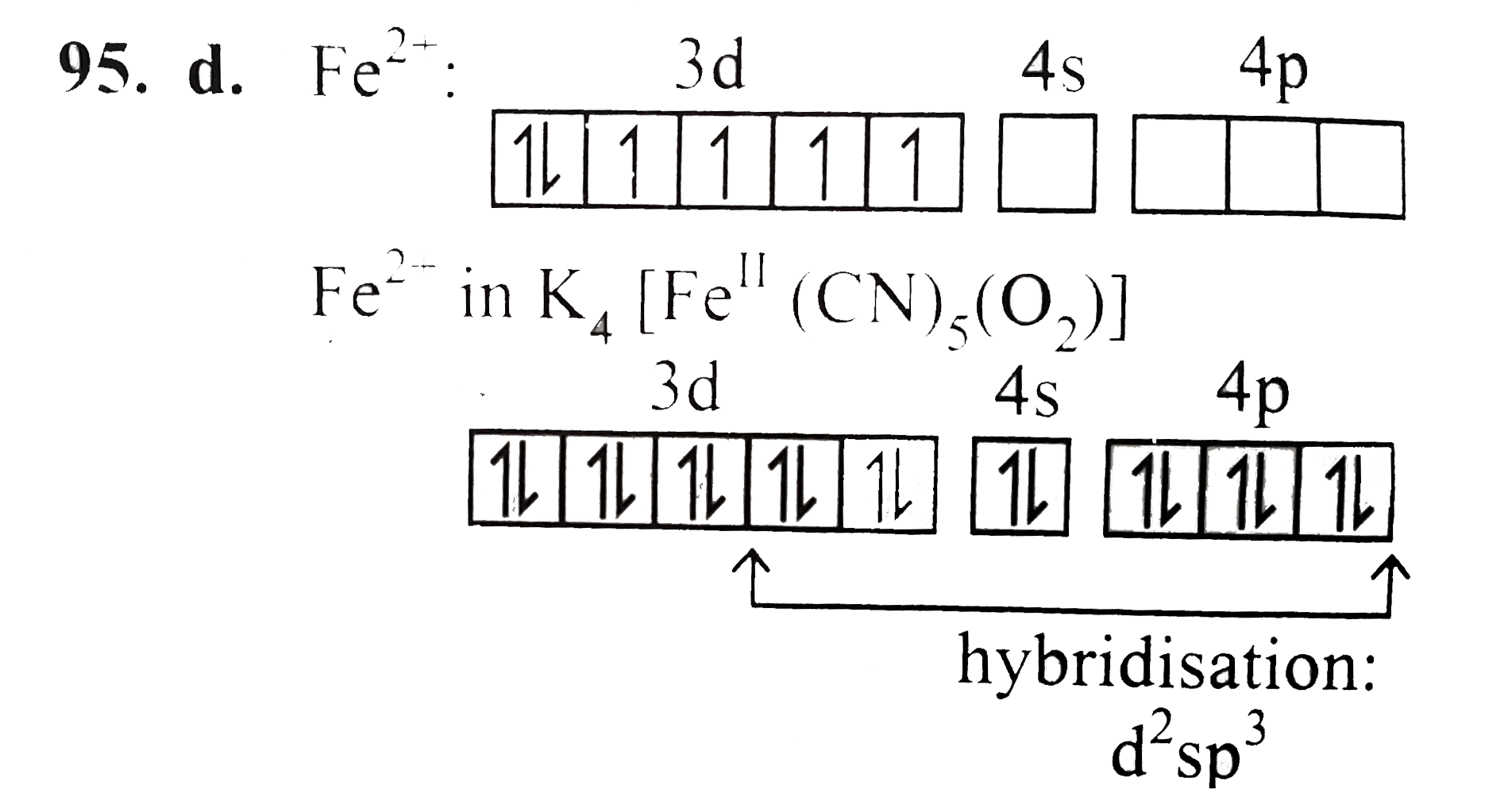A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Application Of Coordination Compounds And Miscellaneous)|29 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|25 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Hybridisation , Magnetic And Optical Properties )|19 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Exercises Single Correct (Crystal Field Theory (Cft))
- Which of the following complex has higher DeltA(0) VALUE?
Text Solution
|
- Relative to the average enerage in the spherical crystal field the t(2...
Text Solution
|
- The crystal field splitting energy for octahedral(Delta(0)) and tetra...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- In which of the following cordination entities, the magnitude of Delta...
Text Solution
|
- In which of the following configuration will there be the possibillity...
Text Solution
|
- For Mn^(3+) ion the electron pairing energy P is about 28,000cm^(1-),D...
Text Solution
|
- Which of the following ligands are correctly represented in an spectro...
Text Solution
|
- The increasing of the crystal field splitting power of some common lig...
Text Solution
|
- Velence bond theroy describes the bonding in complexs in terms of coor...
Text Solution
|
- The complex which has no d-electron in the central metal atom is .
Text Solution
|
- Which of the following statement is correct for the complex Ca(2)[Fe(C...
Text Solution
|
- Which of the folliwing complex is inner orbital as well as low spin co...
Text Solution
|
- The magnetic moment of a complex (A) of Co was found to be 4.89BM and ...
Text Solution
|
- Spin only magnetic moments of a d^(8) ion in octahedral square planar ...
Text Solution
|