Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosAMINES
CENGAGE CHEMISTRY|Exercise QUESTION BANK|1 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-APPENDIX INORGANIC VOLUME 2-Exercises
- Why is the extraction of copper from pyrites more difficult than that ...
Text Solution
|
- Explain: (i). Zone refining (ii). Column chromatography.
Text Solution
|
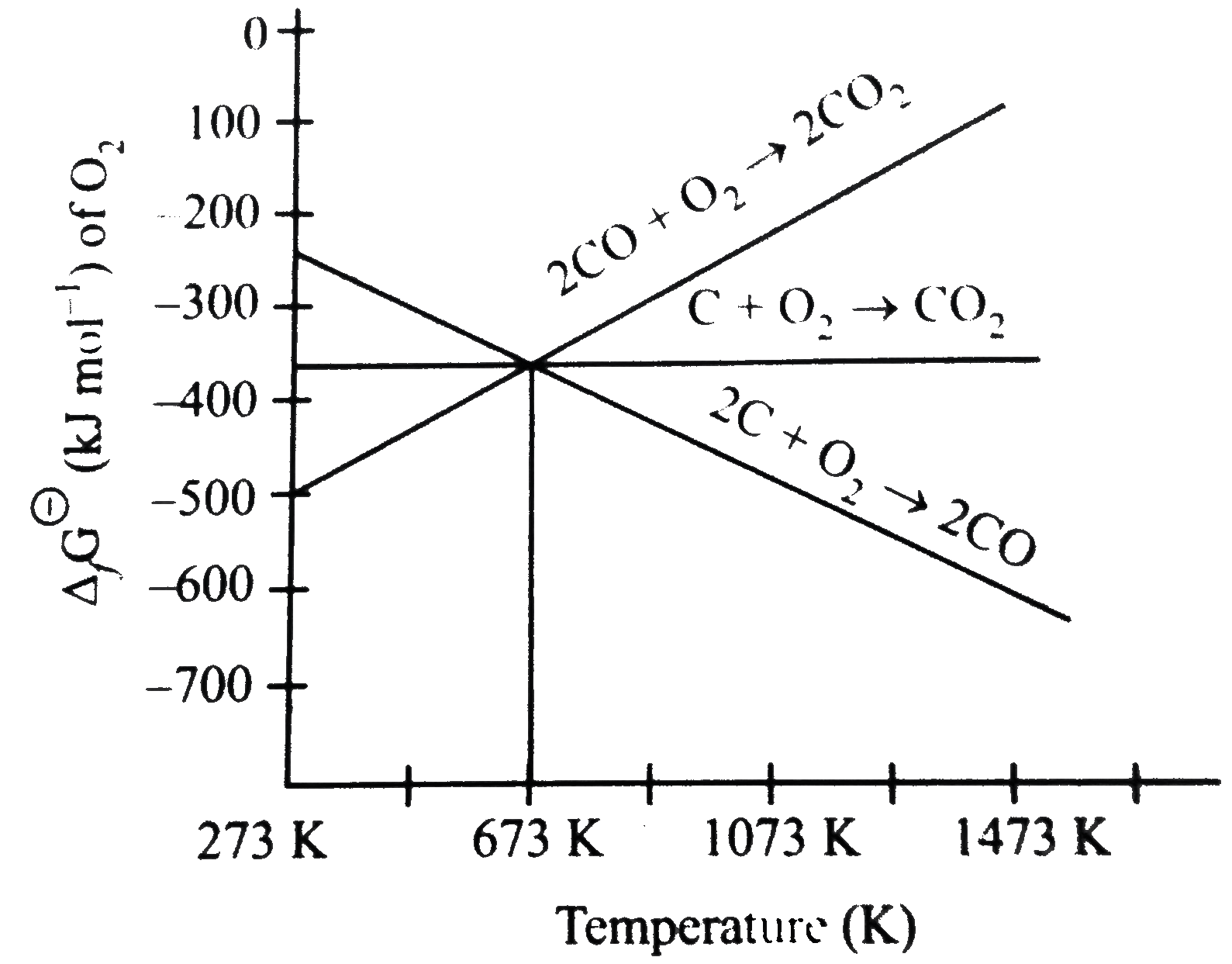
- Out of C and CO, which is a better reducing agent at 673K?
Text Solution
|
- Name the common elements present in the anode mud in electrolytic refi...
Text Solution
|
- Write down the chemical reactions taking place in the extraction of zi...
Text Solution
|
- State the role of silica in the metallurgy of copper.
Text Solution
|
- What is meant by the term "chromatography"?
Text Solution
|
- What criterion is followed for the selectrion of the stationary phase ...
Text Solution
|
- Describe a method for refining nickel.
Text Solution
|
- How can you separate alumina from silica in a bauxite ore associated w...
Text Solution
|
- Giving examples differentiate between roasting and calcination.
Text Solution
|
- How is cast iron different from pig iron?
Text Solution
|
- Differentiate between minerals and ores.
Text Solution
|
- Why copper matte is put in silica lined converter?
Text Solution
|
- What is the role of cryolite in the metallurgy of aluminium?
Text Solution
|
- How is leaching carried out in case of low grade copper ores?
Text Solution
|
- Why is zinc not extracted from zinc oxide through reduction using CO?
Text Solution
|
- The value of triangle(f)G^(ɵ)(Cr2O3)=-540kJmol^-1 and triangle(f)G^(ɵ)...
Text Solution
|
- Out of C and CO, which is a better reducing agent at 673K?
Text Solution
|
- The choice of a reducing agent in a particular case depends on thermod...
Text Solution
|