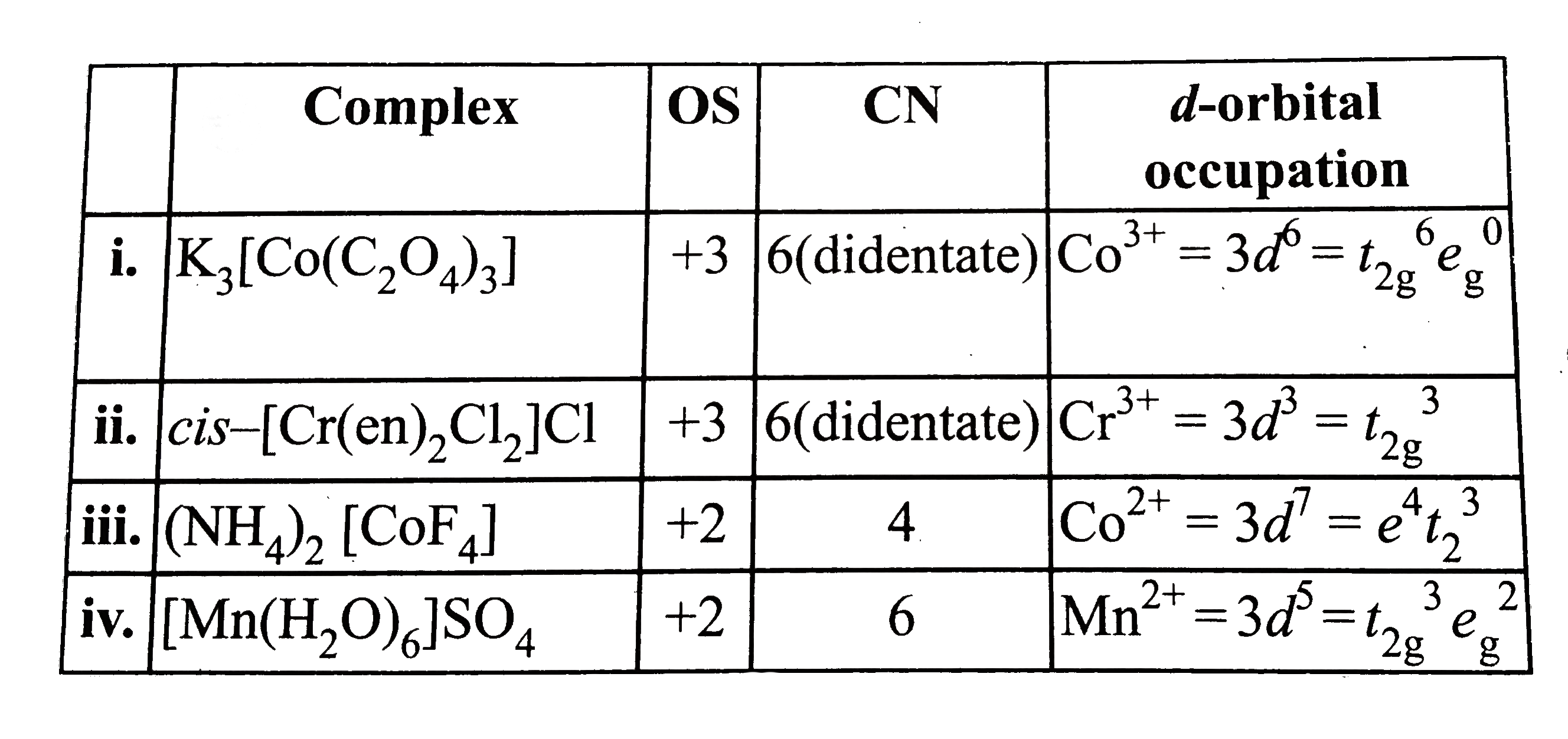Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosAMINES
CENGAGE CHEMISTRY|Exercise QUESTION BANK|1 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-APPENDIX INORGANIC VOLUME 2-Exercises
- Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how man...
Text Solution
|
- Aqueous copper sulphate solution (blue in colour) gives: (i). A gree...
Text Solution
|
- What is the coordination entity formed when excess of aqueous KCN is a...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- What is crystal field splitting energy? How does the magnitude of tria...
Text Solution
|
- What is spectrochemical series?
Text Solution
|
- What is crystal field splitting energy? How does the magnitude of tria...
Text Solution
|
- [Cr(NH3)6]^(3+) is paramagnetic while [Ni(CN)4]^(2-) is diamagnetic. E...
Text Solution
|
- Assertion: A solution of [Ni(H(2)O)(6)]^(2+) is green but a solution o...
Text Solution
|
- [Fe(H2O)6]^(3+) is strongly paramagnetic whereas [Fe(CN)6]^(3-) is wea...
Text Solution
|
- BONDING IN METAL CARBONYLS
Text Solution
|
- Give the oxidation state, d-orbitals occupation and coordination numbe...
Text Solution
|
- Wrtie down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- STABILITY OF COORDINATION COMPOUND
Text Solution
|
- What is meant by the chelate effect? Give and example.
Text Solution
|
- How many ions are produced from the complex Co(NH3)6Cl2 in solution? ...
Text Solution
|
- Amongst the following ions which one has the highest magnetic moment v...
Text Solution
|
- The oxidation number of hyrogen is (i) 0 (ii) +1 (iii) -1 ...
Text Solution
|
- Among the following, the most stable complex is (i). [Fe(H2O)6]^(3+)...
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|