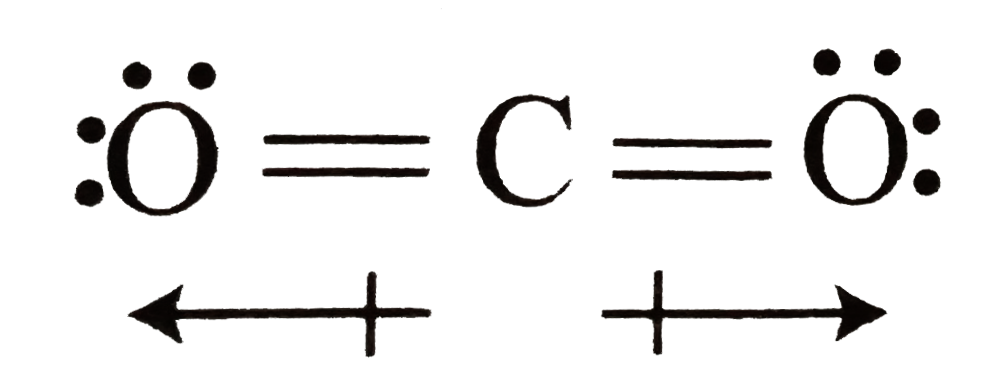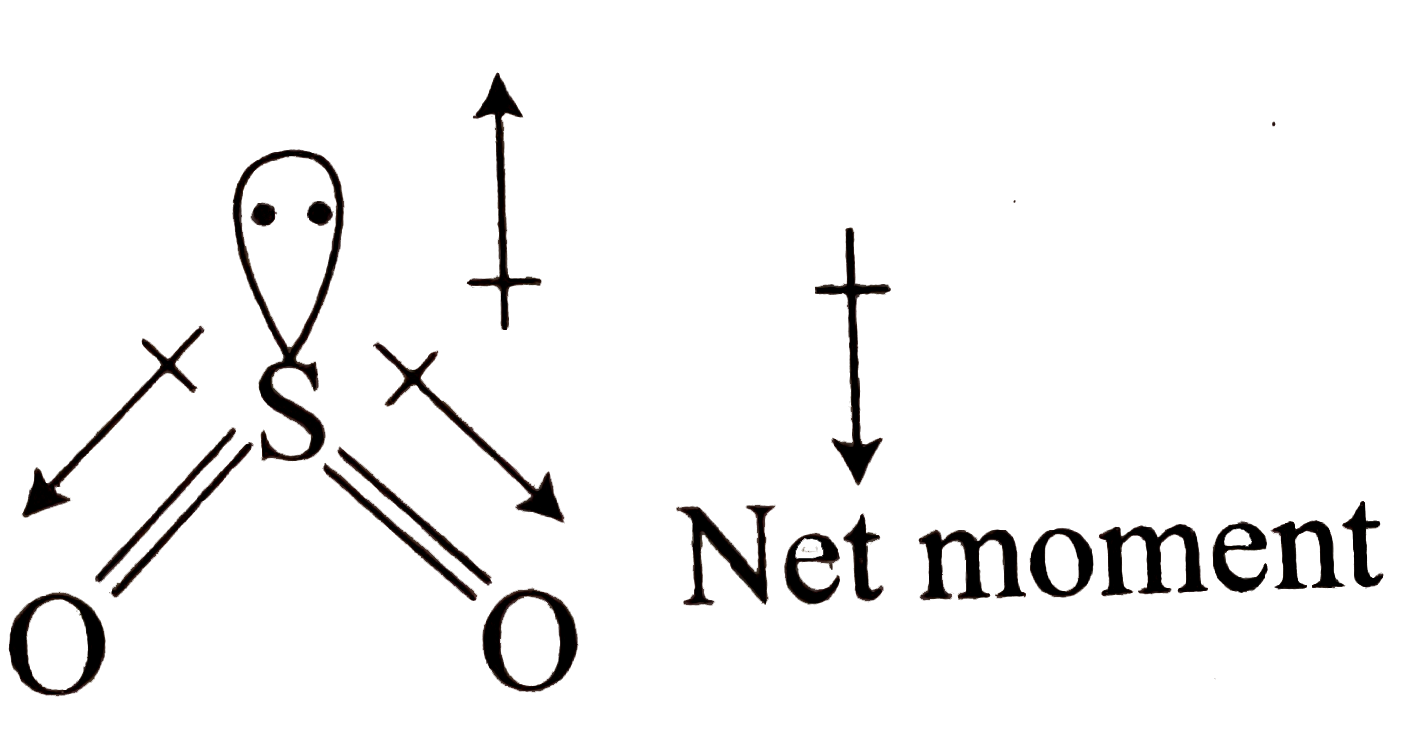(Fig) Lone pair electrons :
(a) `NH_3` and
(b) `NF_3`
In `NH_3`, the net moment of `(N - N)` bonds and the contribution from the `LP overline e^, s` (lone pair `overline e^, s`) are in the same direction and are additive [Fig].The net moment of the `(N - F)` bonds opposes the dipole effect of the `LP overline e^,s` in `NH_3` and the resultant is less `mu`. So `mu` of `NH_3 gt NF_3`.
(ii) The electronegativities of halogens decrease form `F` to `I`, so `mu` of `HG gt HC1 gt HBr gt H1`. But `mu` of `CH_3 F` is smaller than `CH_3 C1` due to shorter `(C - F)` bond distance, although `EN` of `F` is greater than that of `C1`.
(iii) In `CO_2, C` is `sp` hybridised and linear. The bond moments of `(C - O)` are equal and in opposite directions and cancel each other. Hence, `mu` is zero.
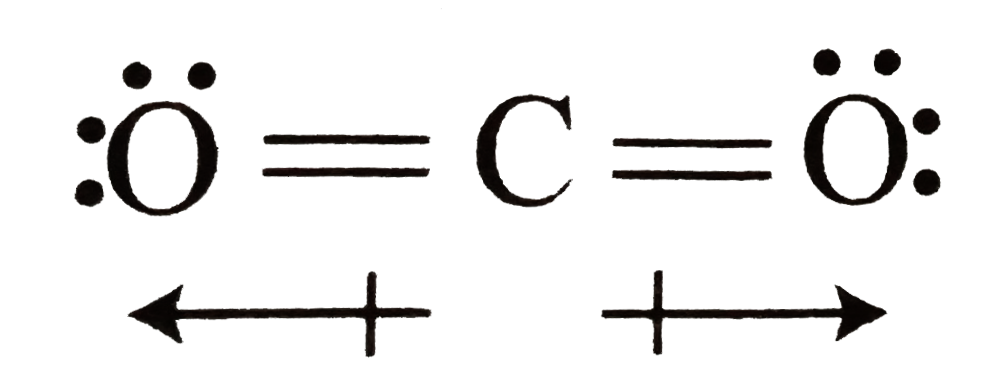
In `SO_2, S` is `sp^2` hybridised having one `LP overline e` one `S` atom. The `(O - S - O)` bond angle is nearly `120^@ , (S - O)` bond moment does not cancel and shown a net resultant `mu`.
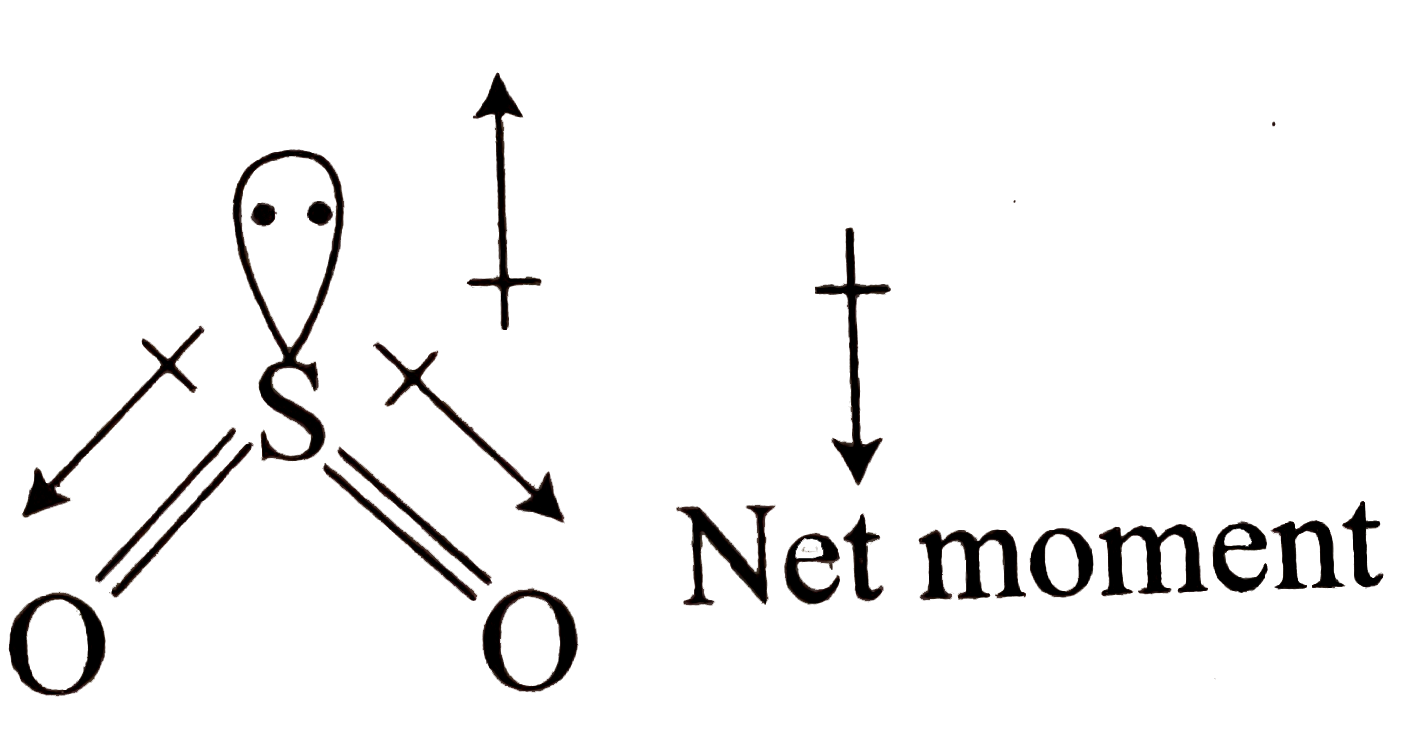
(iv) The `92^@` bond angle suggests that `P` uses three `p` atomic orbitals in forming bonds with `H`, with one `LP overline e` in `3 s` atomic orbital, i.e., `P` in `PH_3` is `sp^2` hybridised (unlike `NH_3`, in which `N` is `sp^3` hybridised).
Therefore, due to the presence of `LP overline e^, s` in `3 s` atomic orbitial of `P`, which is spherical symmetrical, the polarity of the molecule is not affected. In order to affect the polarity of the molecule, `EN` of `P` and `H` are nearly same, so `PH_3` molecule is almost nonpolar.
(v)
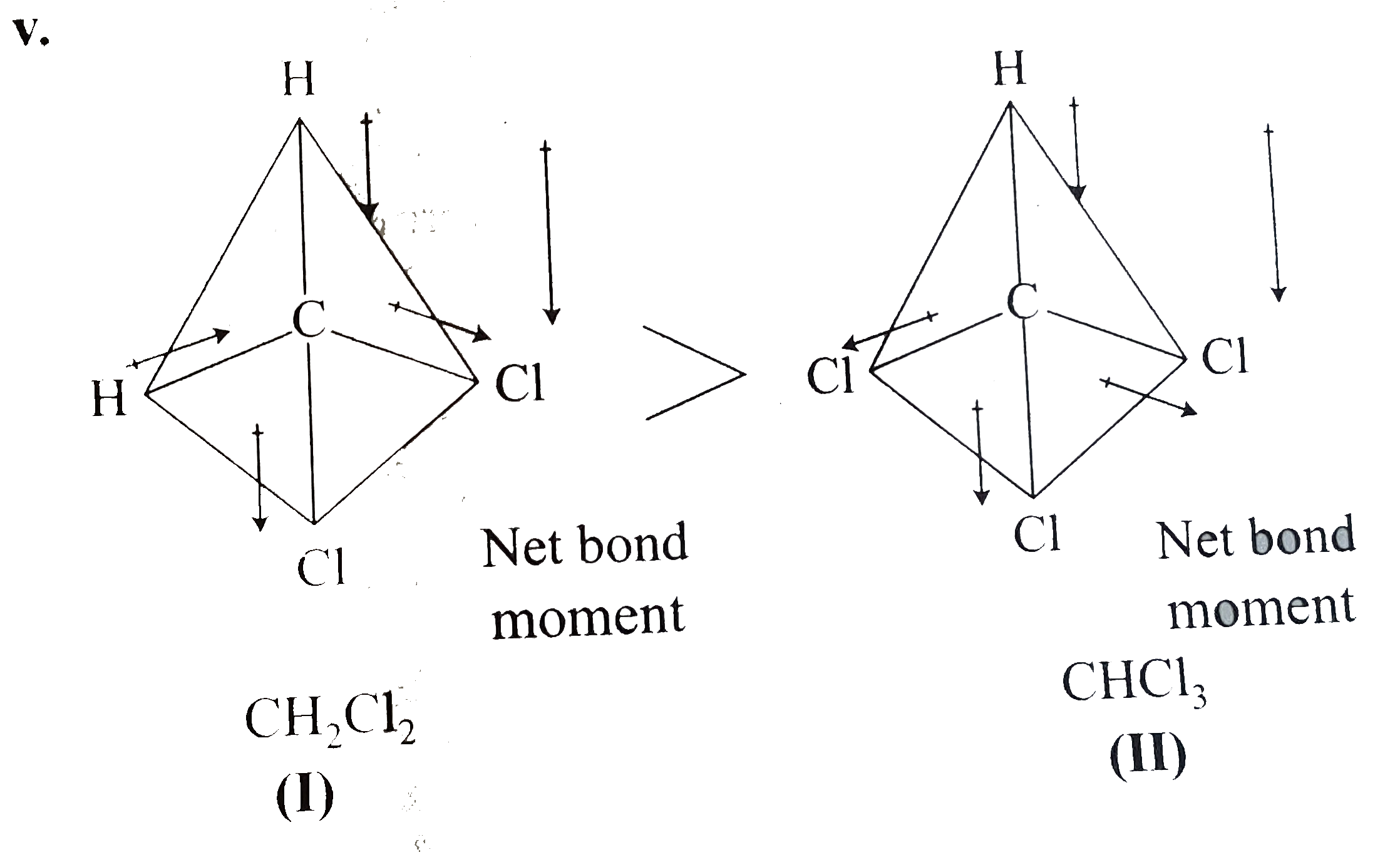
In (I), all bond moments are in the same direction, so they are additive and the net resultant `mu` is more than (II).
In (II), the bond moment of one of the `C1` atoms opposes the net moment of the other two, so the net resultant `mu` is less than that of (I).