A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Single Correct|25 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning|5 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Comprehension|20 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GENERAL ORGANIC CHEMISTRY-Multiple Correct
- Which of the following statement is correct ?
Text Solution
|
- In which of the following Delta G decreases if there can be some intra...
Text Solution
|
- Which of the following statement is/are correct ?
Text Solution
|
- Which of the following is a hard acid ?
Text Solution
|
- Which of the following statement is/are correct ?
Text Solution
|
- Which of the following statement (s) is/are correct ?
Text Solution
|
- Which of the following group (s) is/are o- and p-directing ?
Text Solution
|
- Which of the following group (s) is/are m-directing ?
Text Solution
|
- Which of the following are nucleophiles ?
Text Solution
|
- Which of the following are electrophiles ?
Text Solution
|
- Which of the following have +M effect (overline e - donating mesomeric...
Text Solution
|
- Which of the following have -M effect (overline e - withdrawing mesome...
Text Solution
|
- Which of the following statement (s) is/are correct ?
Text Solution
|
- Which of the following statemenet (s) is/are correct ?
Text Solution
|
- Which of the following statement is/are correct ?
Text Solution
|
- Which of the following statement (s) is/are correct ?
Text Solution
|
- Which benzene sulphuric acid and p-nitrophenol are treated with NaHCO3...
Text Solution
|
- The decreasing order of pKa value of the following is : (I) CH-=CH ...
Text Solution
|
- Among the following which is correct ?
Text Solution
|
- Which one (s) is/are true ?
Text Solution
|
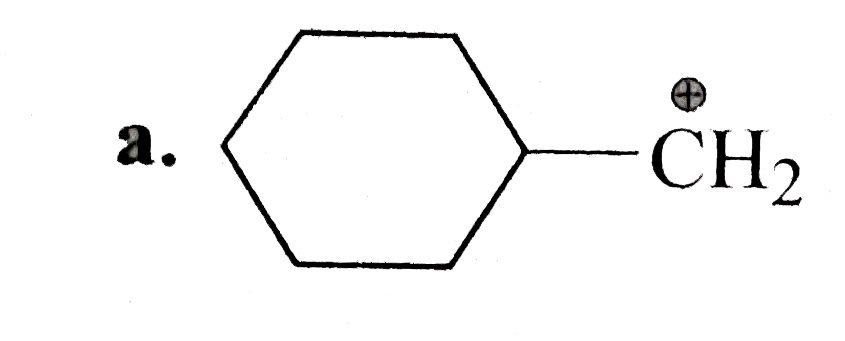
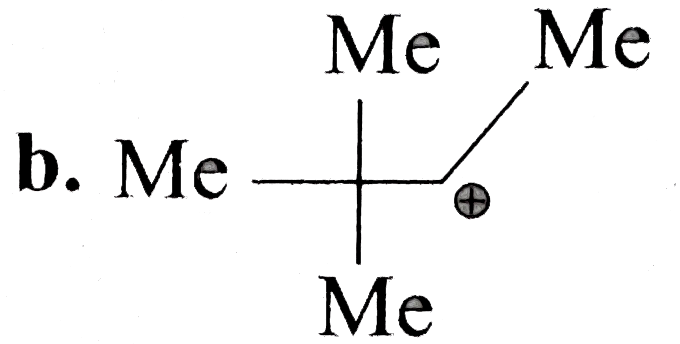
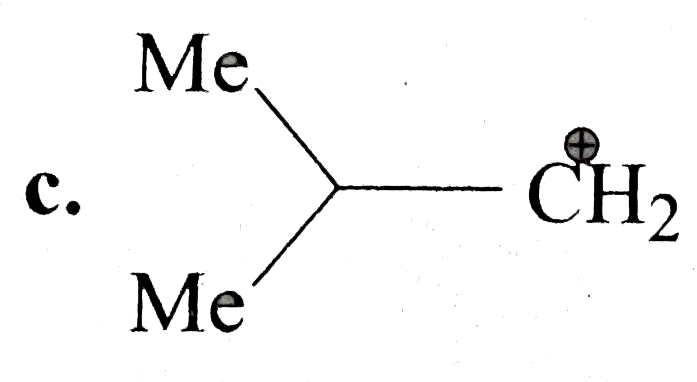
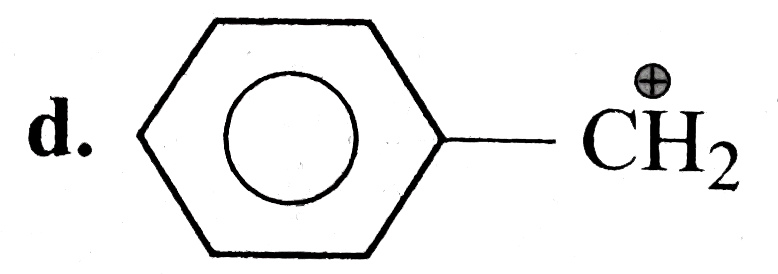
 .
.