(a) Show by enthalpy diagram the rate of solvolysis of `t-BuCl` on increasing the polarity of solvent.
(b) Give the equation of methanolysis of `[Me_3CSMe_(2)]^(oplus) Br^(Ө)`.
(c) Does the effect of solvent prevail for solvolysis of `[Me_3CSMe_(2)]^(oplus)` ?
(a) Show by enthalpy diagram the rate of solvolysis of `t-BuCl` on increasing the polarity of solvent.
(b) Give the equation of methanolysis of `[Me_3CSMe_(2)]^(oplus) Br^(Ө)`.
(c) Does the effect of solvent prevail for solvolysis of `[Me_3CSMe_(2)]^(oplus)` ?
(b) Give the equation of methanolysis of `[Me_3CSMe_(2)]^(oplus) Br^(Ө)`.
(c) Does the effect of solvent prevail for solvolysis of `[Me_3CSMe_(2)]^(oplus)` ?
Text Solution
Verified by Experts
(a) 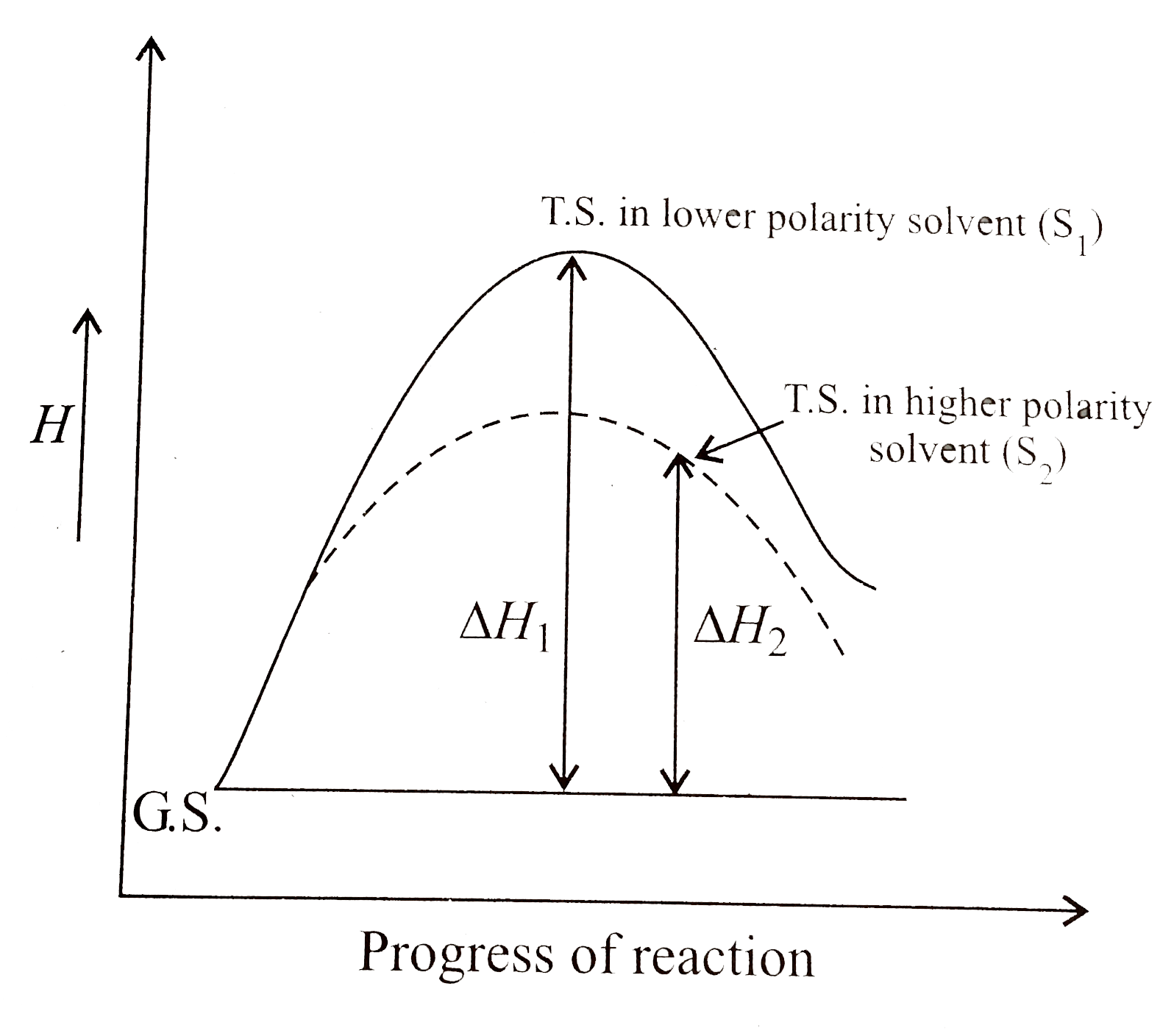
(b)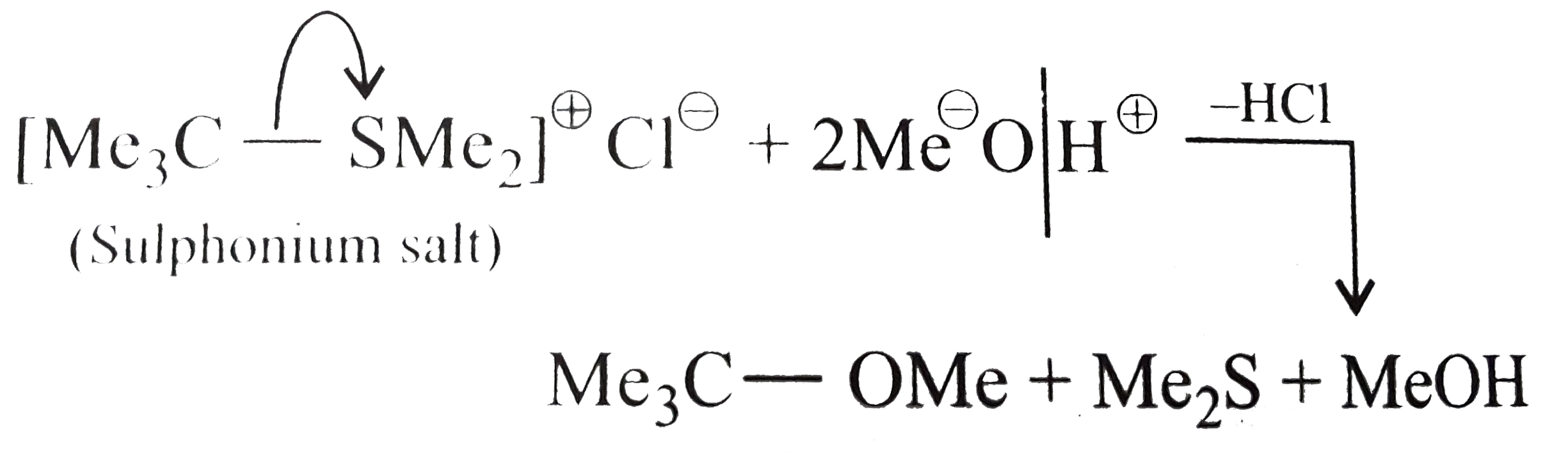 .
.
(c) No effect of solvent. The `G.S` sulphonium cation has a full positive charge. `H_(GS)` is lowered more than `H_(T.S)`. Since in the `T.S`., the positive charge is more diffused, the rate decreases with increase of polarity of solvent [unlike in (a)].
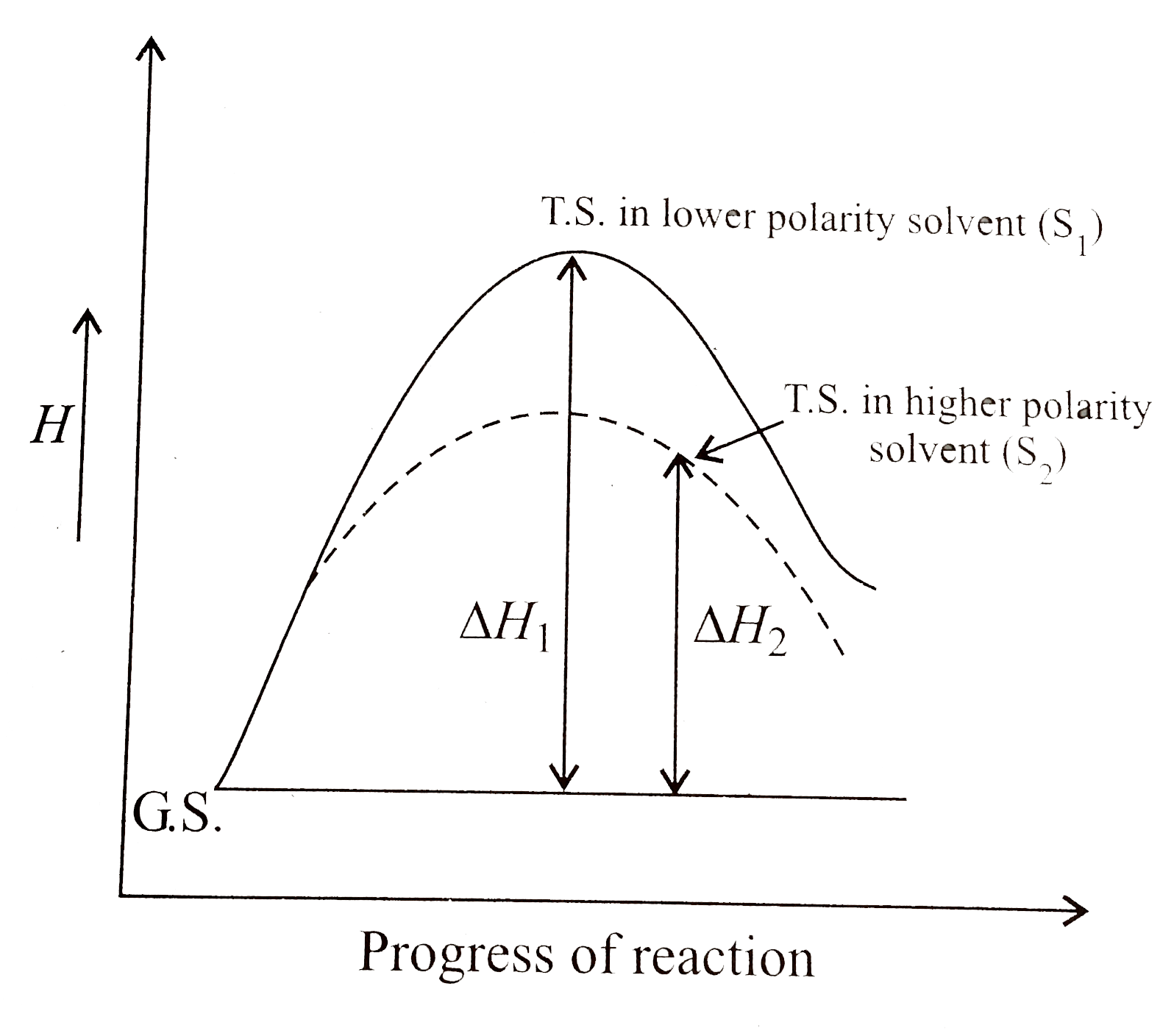
(b)
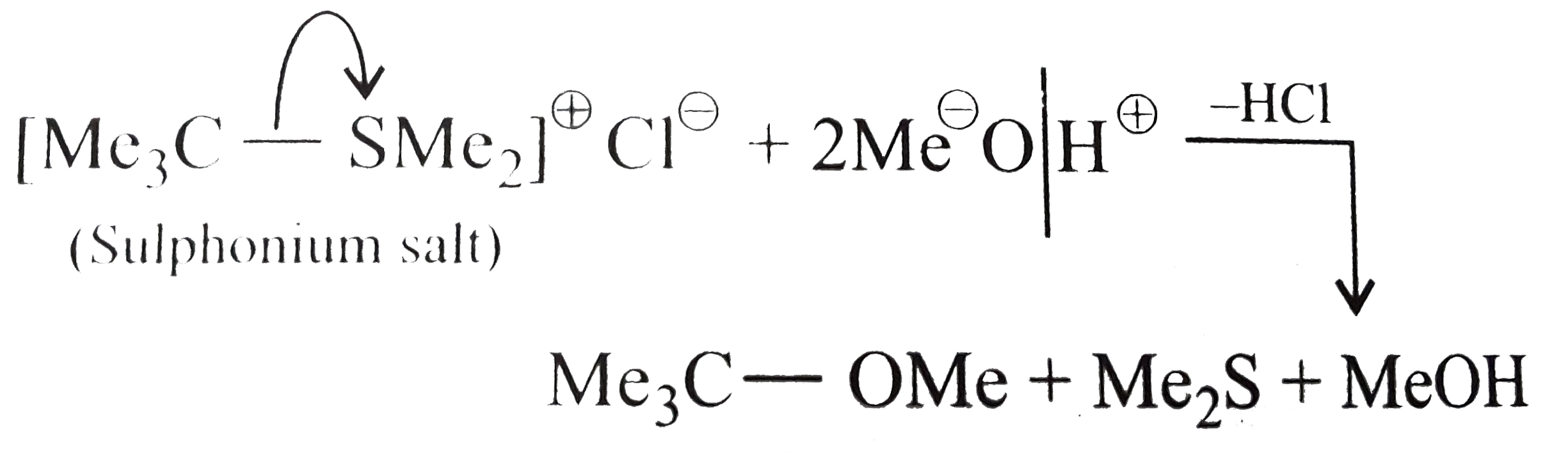 .
. (c) No effect of solvent. The `G.S` sulphonium cation has a full positive charge. `H_(GS)` is lowered more than `H_(T.S)`. Since in the `T.S`., the positive charge is more diffused, the rate decreases with increase of polarity of solvent [unlike in (a)].
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Subjective|19 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Comprehension|36 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|6 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Chemical Equilibrium|72 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
Explain the effect of SN^2 rates for the following reactions : (a) Increasing the polarity of solvent. (b) Show the T.S with all partial charges for each reaction type. (I) Me_2 overset (oplus) S - Me + NH_3 rarr Me overset (oplus)N H_3 + Me_2 S (II) Me I + NH_3 rarr Me overset (oplus)N H_3 + I^(Ө) (III) Me I + overset (Ө) O H rarr Me OH + I^(Ө) (IV) Me_2 S^(oplus) - Me + overset (Ө) OH rarr Me OH + Me_2 S .
(a) Compare the reactivity of Me_3CO^(Ө)K^(oplus) and EtNH_2 in E2 reaction. (b) Why is Me_3CO^(Ө)K^(oplus) superior to EtO^(Ө) in E2 reaction ? (c) Compare the effectiveness of Me_3CO^(Ө)K^(oplus) in DMSO and Me_3COH as solvents. (d) Me_2C C1C H_2 CH_3 overset (Me_3 CO^(Ө)K^*)rarr Major alkene.
Predict the effect of increasing the percentage of H_2 O in acetone -H_2 O solvent mixture in : (a) SN^1 solvolysis of Me_3 C - Br (b) SN^2 reaction of K1 and EtC1 . (c) Predict the relative yields of the product of Me_3 CBr in 80% EtOH and 20 % H_2 O .
(a) Give the Claisen ester condensation product of ethyl butanoate. (b) Why does the Claisen ester condensation of ethyl-2-methyl propanoate (I) not take place ? ( c) Why does Claisen condensation of (I) take place by the use of Ph_3 C^(Ө) Na^(oplus) as a base ?
Write increasing order of basic strength of following: (a)NH_(3)" "(b)MeNH_(2) (c)Me_(2)NH" "(d) Me_(3)N (" in" H_(2)O)
Read the passage given below and answer the following questions: Nucleophilic substitution reaction of haloalkane can be conducted according to both S_(N)^(1) and S_(N)^(2) mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent. Influences of halogen : No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is , the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom, that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I^(-) lt Br^(-) lt Cl^(-) lt F^(-) and the order of their leaving tendency should be I^(-) gt Br^(-) gt Cl^(-) gt F^(-) . Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI gt RBr gt RCl gt RF . In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on S_(N)^(1) mechanism. If the leaving group is not easy to leave, the reaction is based on S_(N)^(2) mechanism. Influences of solvent polarity: In S_(N)^(1) reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In S_(N)^(2) reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (S_(N)^(1)) of tertiary chlorobutane in 25^(@)C water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (S_(N)^(2)) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both S_(N)^(1) and S_(N)^(2) reactions, but with different results. Generally speaking, weak polar solvent is favorable for S_(N)^(2) reaction, while strong polar solvent is favorable for S_N^(1) reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on S_(N)^(1) mechanism in solvents with a strong polarity (for example, ethanol containing water). (Ding, Y. (2013). A Brief Discussion on Nucleophilic Substitution Reaction on Saturated Carbon Atom. In Applied Mechanics and Materials (Vol. 312, pp. 433-437). Trans Tech Publications Ltd.) S_(N)^(1) mechanism is favoured in which of the following solvents:
Read the passage given below and answer the following questions: Nucleophilic substitution reaction of haloalkane can be conducted according to both S_(N)^(1) and S_(N)^(2) mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent. Influences of halogen : No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is , the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom, that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I^(-) lt Br^(-) lt Cl^(-) lt F^(-) and the order of their leaving tendency should be I^(-) gt Br^(-) gt Cl^(-) gt F^(-) . Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI gt RBr gt RCl gt RF . In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on S_(N)^(1) mechanism. If the leaving group is not easy to leave, the reaction is based on S_(N)^(2) mechanism. Influences of solvent polarity: In S_(N)^(1) reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In S_(N)^(2) reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (S_(N)^(1)) of tertiary chlorobutane in 25^(@)C water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (S_(N)^(2)) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both S_(N)^(1) and S_(N)^(2) reactions, but with different results. Generally speaking, weak polar solvent is favorable for S_(N)^(2) reaction, while strong polar solvent is favorable for S_N^(1) reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on S_(N)^(1) mechanism in solvents with a strong polarity (for example, ethanol containing water). (Ding, Y. (2013). A Brief Discussion on Nucleophilic Substitution Reaction on Saturated Carbon Atom. In Applied Mechanics and Materials (Vol. 312, pp. 433-437). Trans Tech Publications Ltd.) Nucleophilic substitution will be fastest in case of:
Read the passage given below and answer the following questions: Nucleophilic substitution reaction of haloalkane can be conducted according to both S_(N)^(1) and S_(N)^(2) mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent. Influences of halogen : No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is , the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom, that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I^(-) lt Br^(-) lt Cl^(-) lt F^(-) and the order of their leaving tendency should be I^(-) gt Br^(-) gt Cl^(-) gt F^(-) . Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI gt RBr gt RCl gt RF . In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on S_(N)^(1) mechanism. If the leaving group is not easy to leave, the reaction is based on S_(N)^(2) mechanism. Influences of solvent polarity: In S_(N)^(1) reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In S_(N)^(2) reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (S_(N)^(1)) of tertiary chlorobutane in 25^(@)C water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (S_(N)^(2)) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both S_(N)^(1) and S_(N)^(2) reactions, but with different results. Generally speaking, weak polar solvent is favorable for S_(N)^(2) reaction, while strong polar solvent is favorable for S_N^(1) reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on S_(N)^(1) mechanism in solvents with a strong polarity (for example, ethanol containing water). (Ding, Y. (2013). A Brief Discussion on Nucleophilic Substitution Reaction on Saturated Carbon Atom. In Applied Mechanics and Materials (Vol. 312, pp. 433-437). Trans Tech Publications Ltd.) S_(N)^(1) reaction will be fastest in which of the following solvents?
Read the passage given below and answer the following questions: Nucleophilic substitution reaction of haloalkane can be conducted according to both S_(N)^(1) and S_(N)^(2) mechanisms. However, which mechanism it is based on is related to such factors as the structure of haloalkane, and properties of leaving group, nucleophilic reagent and solvent. Influences of halogen : No matter which mechanism the nucleophilic substitution reaction is based on, the leaving group always leave the central carbon atom with electron pair. This is just the opposite of the situation that nucleophilic reagent attacks the central carbon atom with electron pair. Therefore, the weaker the alkalinity of leaving group is , the more stable the anion formed is and it will be more easier for the leaving group to leave the central carbon atom, that is to say, the reactant is more easier to be substituted. The alkalinity order of halogen ion is I^(-) lt Br^(-) lt Cl^(-) lt F^(-) and the order of their leaving tendency should be I^(-) gt Br^(-) gt Cl^(-) gt F^(-) . Therefore, in four halides with the same alkyl and different halogens, the order of substitution reaction rate is RI gt RBr gt RCl gt RF . In addition, if the leaving group is very easy to leave, many carbocation intermediates are generated in the reaction and the reaction is based on S_(N)^(1) mechanism. If the leaving group is not easy to leave, the reaction is based on S_(N)^(2) mechanism. Influences of solvent polarity: In S_(N)^(1) reaction, the polarity of the system increases from the reactant to the transition state, because polar solvent has a greater stabilizing effect on the transition state than the reactant, thereby reduce activation energy and accelerate the reaction. In S_(N)^(2) reaction, the polarity of the system generally does not change from the reactant to the transition state and only charge dispersion occurs. At this time, polar solvent has a great stabilizing effect on Nu than the transition state, thereby increasing activation energy and slow down the reaction rate. For example, the decomposition rate (S_(N)^(1)) of tertiary chlorobutane in 25^(@)C water (dielectric constant 79) is 300000 times faster than in ethanol (dielectric constant 24). The reaction rate (S_(N)^(2)) of 2-bromopropane and NaOH in ethanol containing 40% water is twice slower than in absolute ethanol. In a word, the level of solvent polarity has influence on both S_(N)^(1) and S_(N)^(2) reactions, but with different results. Generally speaking, weak polar solvent is favorable for S_(N)^(2) reaction, while strong polar solvent is favorable for S_N^(1) reaction, because only under the action of polar solvent can halogenated hydrocarbon dissociate into carbocation and halogen ion and solvents with a strong polarity is favorable for solvation of carbocation, increasing its stability. Generally speaking, the substitution reaction of tertiary haloalkane is based on S_(N)^(1) mechanism in solvents with a strong polarity (for example, ethanol containing water). (Ding, Y. (2013). A Brief Discussion on Nucleophilic Substitution Reaction on Saturated Carbon Atom. In Applied Mechanics and Materials (Vol. 312, pp. 433-437). Trans Tech Publications Ltd.) Polar solvents make the reaction faster as they:
CENGAGE CHEMISTRY-ORGANIC REACTION MECHANISM-Solved Example
- (a) Explain how the sterochemistry of SN^1 and SN^2 differs. (b) Exp...
Text Solution
|
- (a) Define dielectric constant. (b) How does dielectric constant aff...
Text Solution
|
- (a) Show by enthalpy diagram the rate of solvolysis of t-BuCl on incre...
Text Solution
|
- Hydrolysis of 2-bromo-3-methyl butane (2^@) yields only 2-methy-2-buta...
Text Solution
|
- Write structure for the solvolysis of Et3 C-Cl with : (a) MeOH (b)...
Text Solution
|
- Explain the relative rates of RX with H2 O/EtOH at 25^@ C as given : ...
Text Solution
|
- Predict the order of nucleophicity of X^(Ө) in the following reaction ...
Text Solution
|
- Give the product of the following displacement reactions : (a) (R )-...
Text Solution
|
- Explain .
Text Solution
|
- When 3-chlorocyclopropene (A) is treated with SbCl5, it gives a stable...
Text Solution
|
- Explain : (a) An aq. Solution to troplylium bormide (C7 H7Br) on tre...
Text Solution
|
- Give the order of reactivity towards aqueous HCOOH of the following. ...
Text Solution
|
- Give the order of hydrolysis in SN^1 process for the following : ...
Text Solution
|
- Give the order of (a) nucleophilicity (b) basic character, and (c) fug...
Text Solution
|
- Give the most reactive substrate in each of the following pair with ov...
Text Solution
|
- Outline the synthesis of following compounds from suitable nucleophile...
Text Solution
|
- Give a suitable mechanism for the following : .
Text Solution
|
- Predict the order of reactivity of the following halides with (a) NaI ...
Text Solution
|
- Explain : (a)ClCH2OCH3 (chloromethyl methyl ether) undergoes SN^1 re...
Text Solution
|
- Write the products of the following SN reactions. .
Text Solution
|