A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Archives|8 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise True/False|2 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Single correct|50 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Chemical Equilibrium|72 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ORGANIC REACTION MECHANISM-Assertion-Reasoning
- 1^@ allylic halides are more reactive than 1^@ RX in SN^1 reaction. ...
Text Solution
|
- Walden inversion takes place in SN^2 reaction. Half-life period of S...
Text Solution
|
- Chlorination of allylic hydrogen is difficult than vinylic hydrogen. ...
Text Solution
|
- Heavy metal ions Ag^+ or Pb^(2+) decrease SN^1 reactivity. They aid ...
Text Solution
|
- Crown ether acts as phase transfer catalysis and increases SN^2 reacti...
Text Solution
|
- RS^(Ө) is a stronger nucleophile and a better leaving group than RO^(Ө...
Text Solution
|
- The product (B) results by SN^2 mechanism and product (c) results by S...
Text Solution
|
- (I) Mel+PH(3)overset(60% "acetone")overset(+40%H(2)O)toMeoverset(oplus...
Text Solution
|
- ElcB reaction is favoured by stabilisation of carbanion and poor leavi...
Text Solution
|
- Rate of ethanolysis of 1^@ halide by SN^1 machanism is fast. Caroca...
Text Solution
|
- Phenol is more reactive than benzene towards electrophilic substitutio...
Text Solution
|
- Addition of bromine to trans-2-butene yields meso-2,3-dibromobutane. ...
Text Solution
|
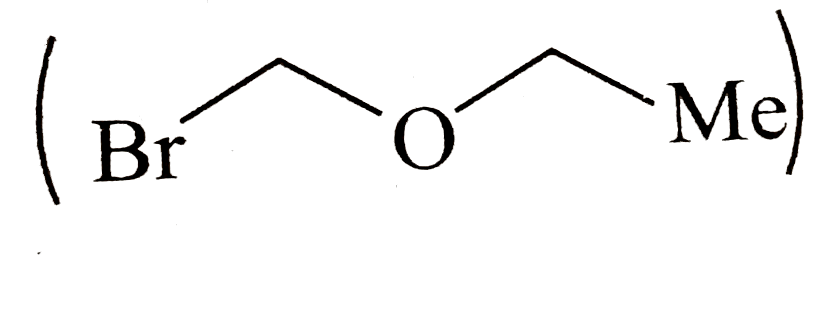 by `SN^1` machanism is fast.
by `SN^1` machanism is fast.