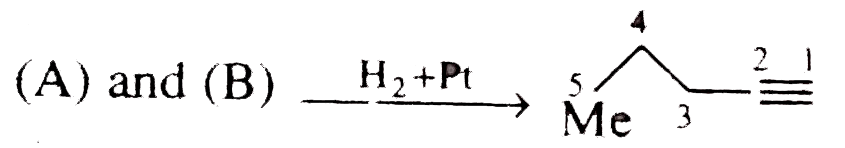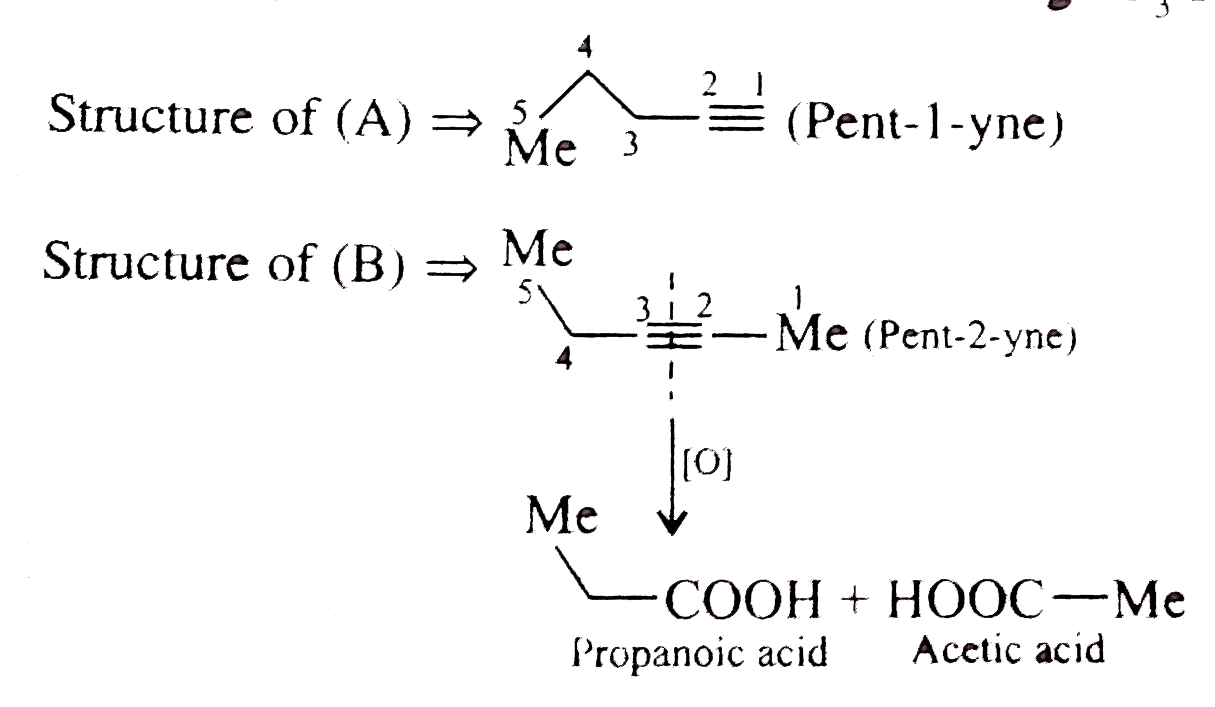Text Solution
Verified by Experts
Topper's Solved these Questions
ALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension Type)|51 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct Answers Type)|25 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Solved Examples|24 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Single correct Answer|14 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALKYNES-Exercises (Subjective Type)
- Identify the three alkynes A, B (CH(10)H(18)), and C (C(10)H(16)) whic...
Text Solution
|
- Deduce the structural fomula of a compound A (C6H(10)) which shows the...
Text Solution
|
- Identify (A) to (E).
Text Solution
|
- Write the structures of isomeric hexynes and also give their IUPAC nam...
Text Solution
|
- What are the geometrices of: i. Prop-1-yne ii. But-2-yne iii. He...
Text Solution
|
- Complete the following:
Text Solution
|
- Identify A, B and C.
Text Solution
|
- Identify the products.
Text Solution
|
- a. Convert but-1-yne to but 2-yne and vice versa. b. Convert (cis or...
Text Solution
|
- Complete the following
Text Solution
|
- Identify the products.
Text Solution
|
- Complete the following reaction
Text Solution
|
- There are two paths to prepare compound (C). Which path is feasib...
Text Solution
|
- Give the structures of reactants:
Text Solution
|
- Give the structure of reactants:
Text Solution
|
- Complete the following equations:
Text Solution
|
- With alcoholic potash, C4H8Cl2 (A) gives C4H6 (B), which reacts with a...
Text Solution
|
- Three compounds A, B, and C are isomers of the formula C5H8. All of th...
Text Solution
|
- A dihalogen derivative (A) of a hydrocarbon having two carbon atoms re...
Text Solution
|
- An unsaturated hydrocarbon (A), C6H(10), readily gives (B) on treatmen...
Text Solution
|