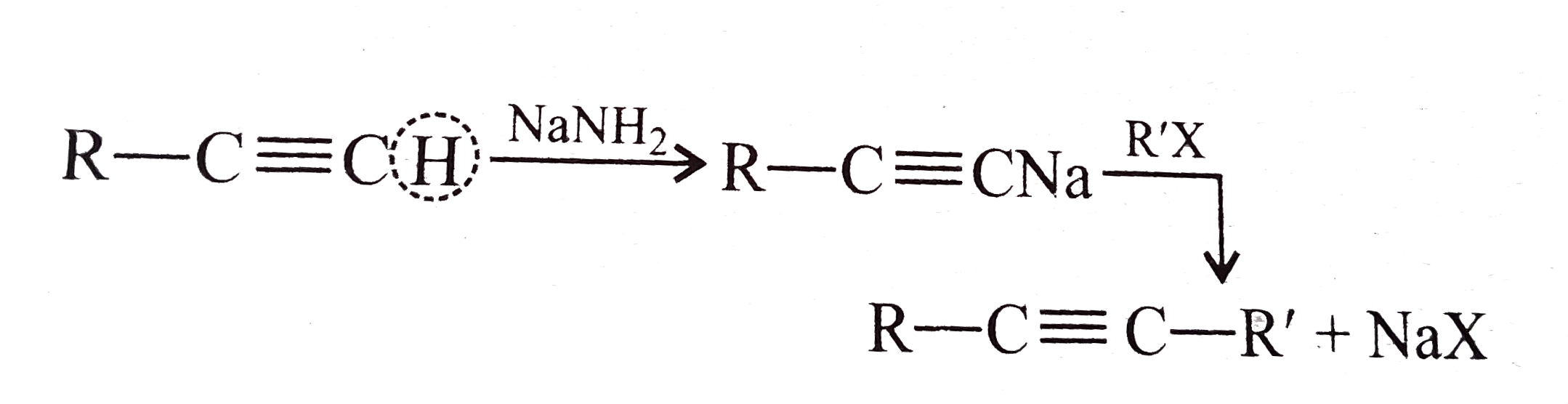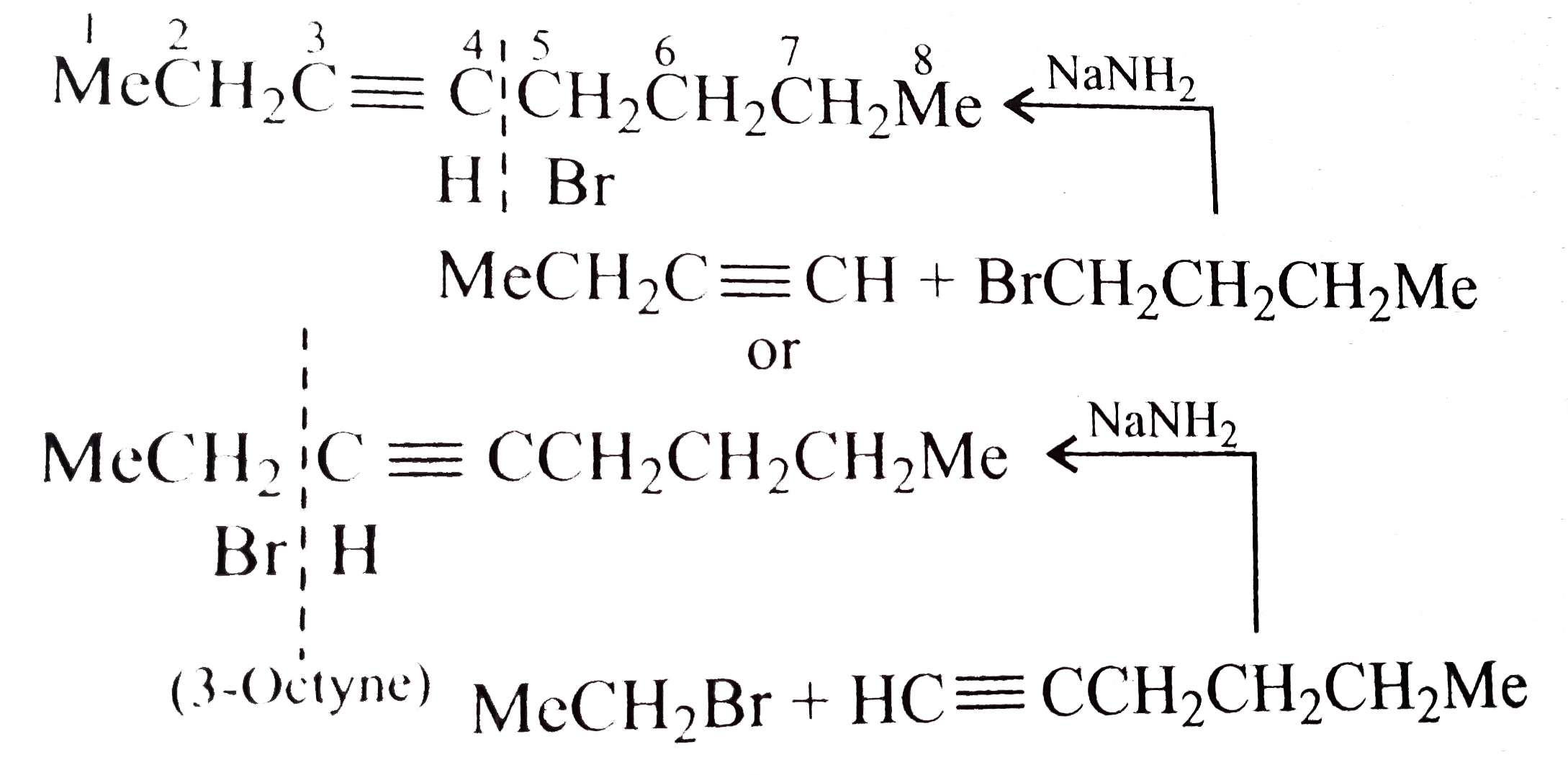A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Fill in the Blanks Type)|3 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct Answers Type)|30 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Single correct Answer|14 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALKYNES-Exercises (Archives - Single Correct Answer Type)
- When propyne is treated with aqueous H2SO4 in the presence of HgSO4, t...
Text Solution
|
- Acidic hydrogen is present in:
Text Solution
|
- The number of stuctural and configuration isomers of a bromo compound,...
Text Solution
|
- Identify a reagent from the following list which can easily distinguis...
Text Solution
|
- The reagent(s) for the following conversion is/are:
Text Solution
|
- The synthesis of 3-octyne is achieved by adding a bromoalkane into a m...
Text Solution
|