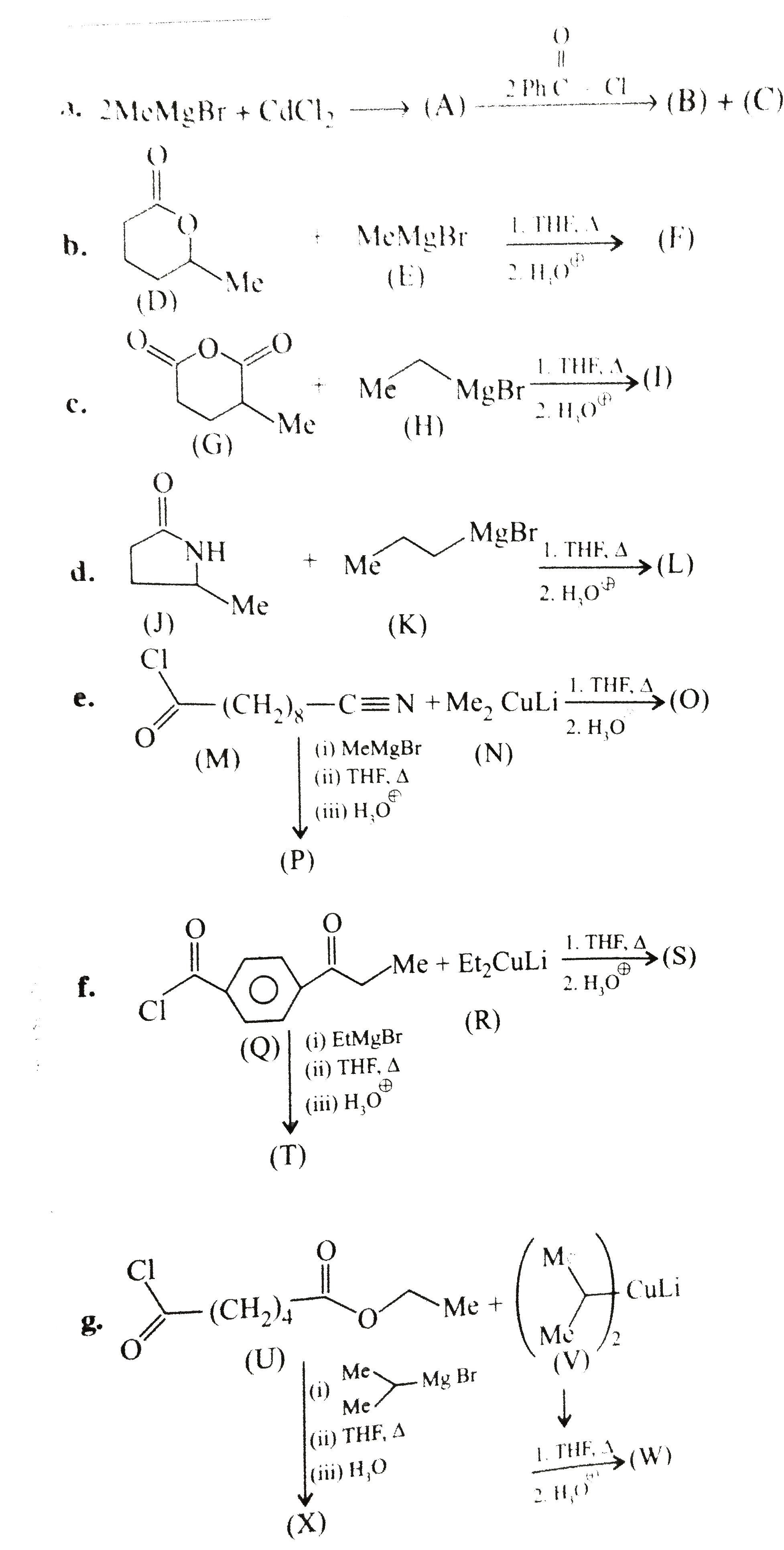
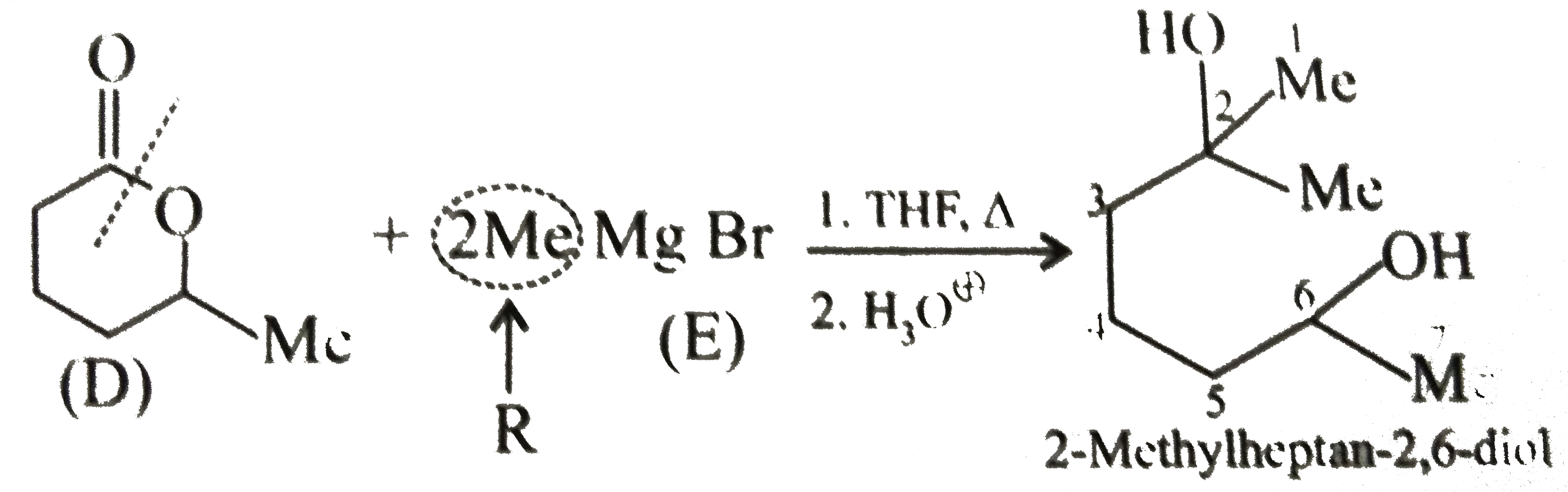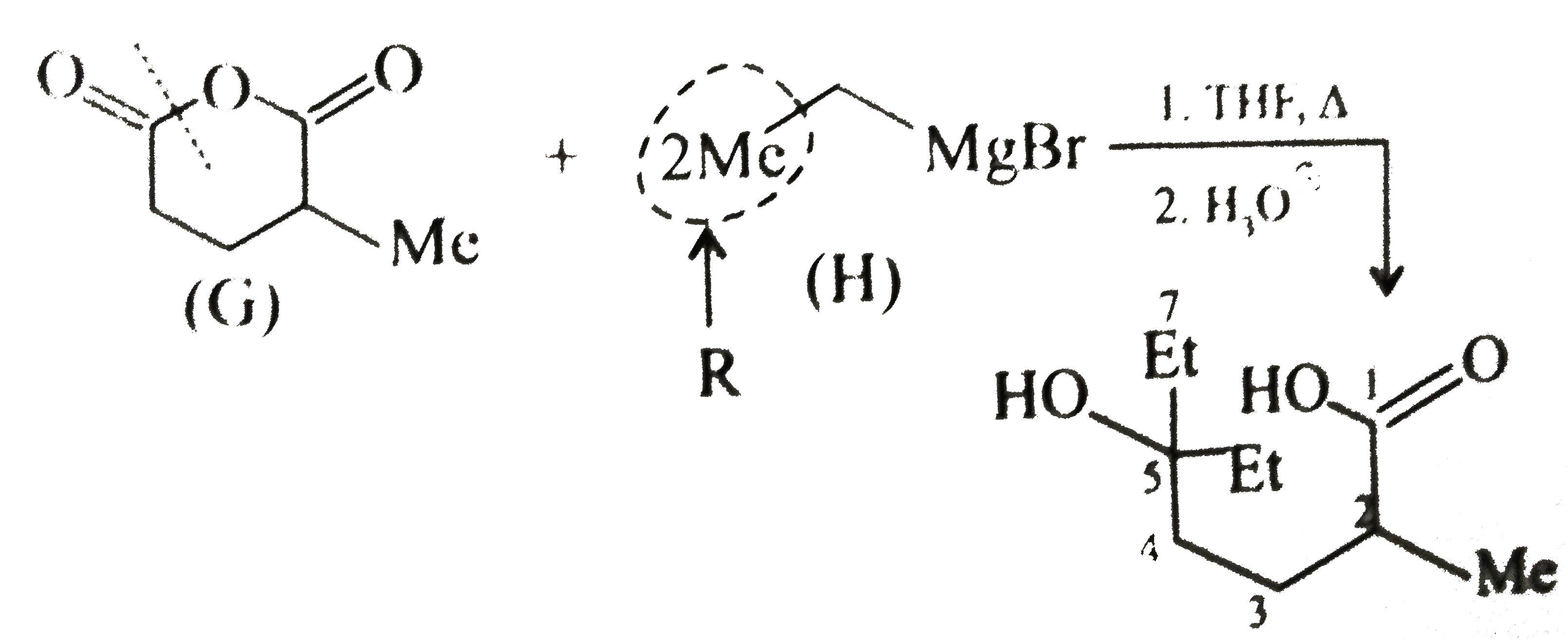(a) `underset("Benzoyl chloride")(2Ph-overset(O)overset(||)(C)-Cl)+underset("cadmium")underset("Dimethyl")(Me_(2)Cd)tounderset((A))(2Ph)-underset("Acetophenone")underset((B))(overset(O)overset(||)(C))-Me+underset("choloride")underset("Cadmium")underset((C))(CdCl_(2))`
(b) `(D)` is a cyclic ester, called lactone. The ester reacts with `2 mol` of `G.R` to give `3^@` alcohol. (Two `R` groups come from the `G.R`)
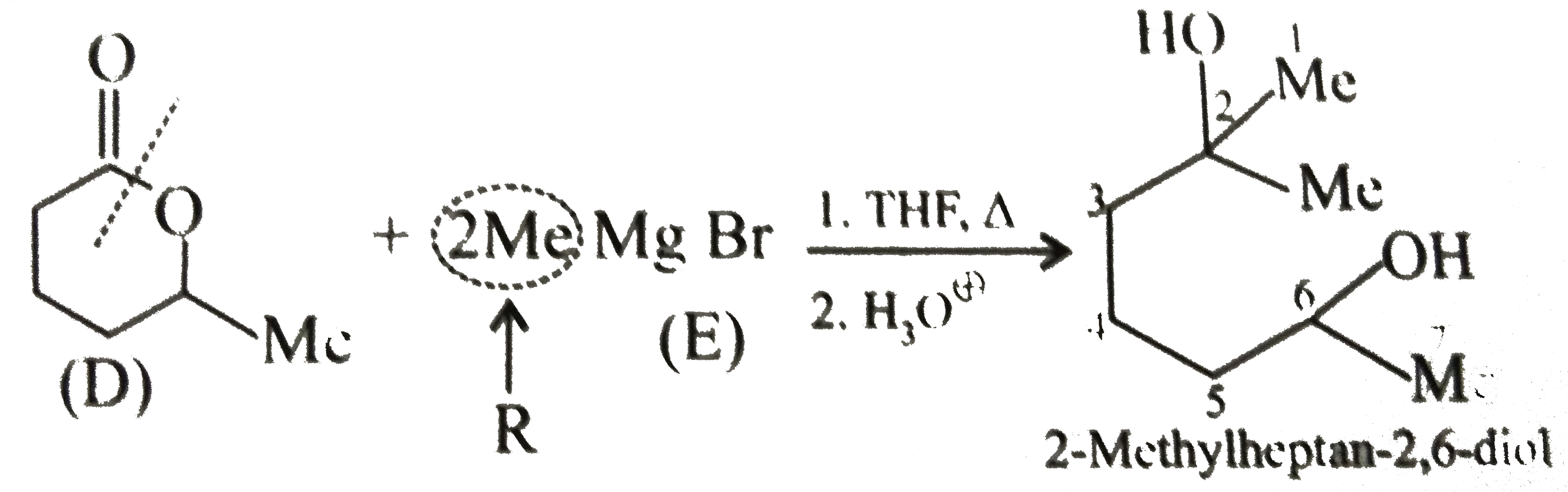
( c) `(G)` is a cyclic anhydride. The anhydride reacts with `2 mol` of `G.R` to give `2^@` alcohol (two `R` groups come from the `G.R`) Nucleophilic addition `(NA)` reaction is favoured by the electron-withdrawing group `(EWG)` and disfavoured by electron-donating group `(EDG)`. The cleavage of ring takes place from the side not containing the `EDG` (Me group).
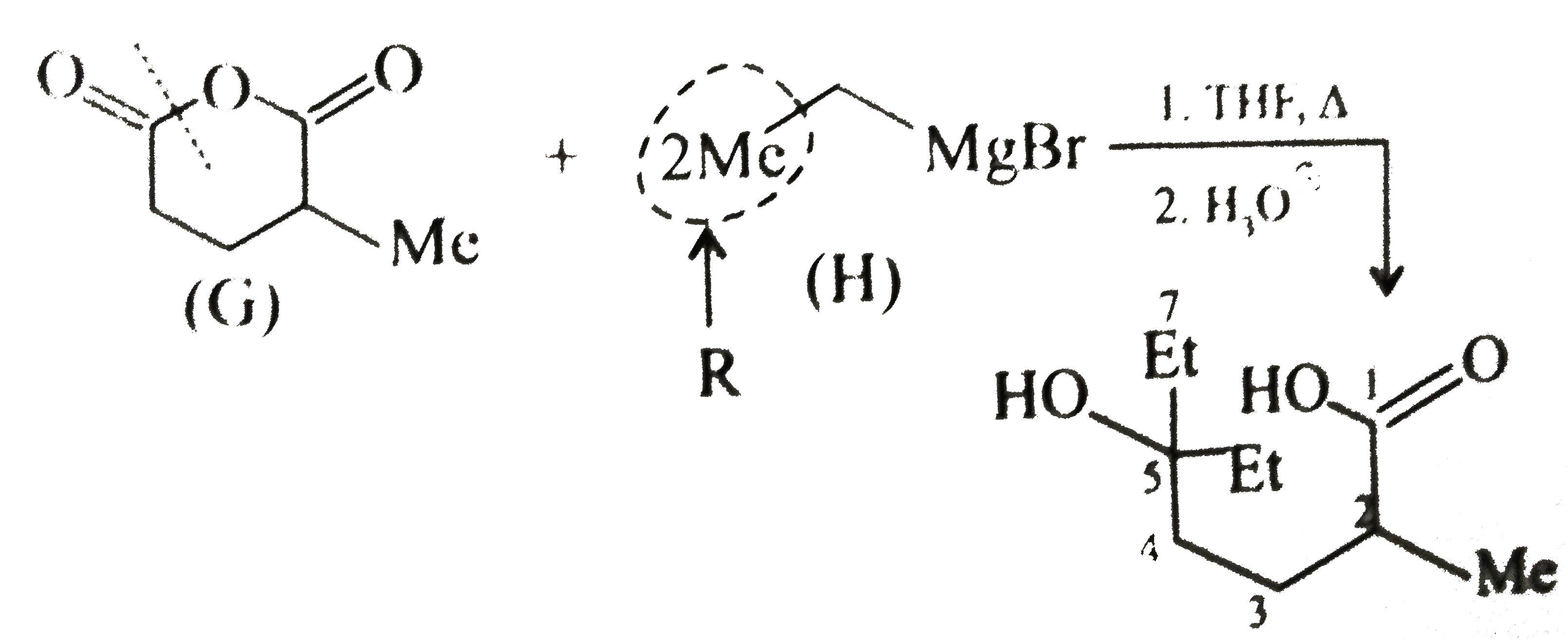
(d) `(J)` is a cyclic amide, called lactam. The amide reacts with `2 mol` of `G.R` to give ketone (one `R` comes from the `G.R`).

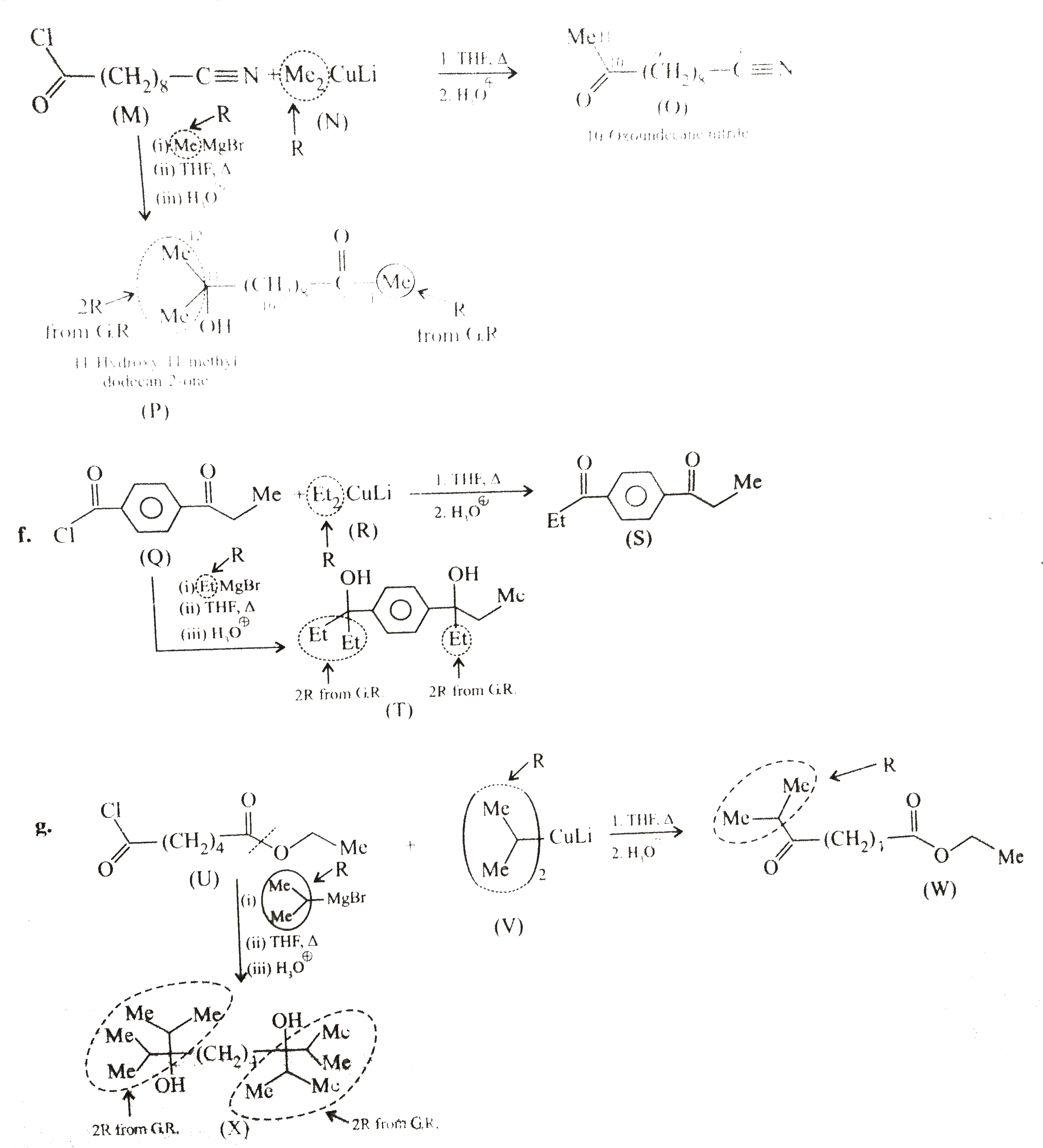
In `(e),(f)` and `(g)`, less reactive `R_2 CuLi` (dialkyl lithium cuprate) reacts only with more reactive `(-overset(O)overset(||)(C)-Cl)`
group, but more reactive `G.R` reacts with both `(-overset(O)overset(||)(C)-Cl)` and `(-C -= N)` groups. Two moles of `G.R` reacts with `(-overset(O)overset(||)(C)-Cl)` group to give `3^@` alcohol (two `R` groups come from the `G.R`) , but with `(-C -= N)` group, the `G.R` gives ketone (one `R` group comes from `G.R`).
(e)