A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|16 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|47 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Subjective )|17 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Exercises (Linked Comprehension)
- The structure of product (F) is :
Text Solution
|
- The structure of product (B) is :
Text Solution
|
- The gas ( C) is :
Text Solution
|
- The structure of product (D) is :
Text Solution
|
- The structure of product (E) is :
Text Solution
|
- The structure of product (F) is :
Text Solution
|
- The structure of product (B) is :
Text Solution
|
- The structure of product (C) is :
Text Solution
|
- The structure of product (D) is :
Text Solution
|
- The structure of product (E) is :
Text Solution
|
- The structure of product (A) is :
Text Solution
|
- The structure of product (B) is :
Text Solution
|
- The structure of product (C) is :
Text Solution
|
- The structure of product (D) is :
Text Solution
|
- The structure of product (E) is :
Text Solution
|
- The structure of product (F) is :
Text Solution
|
- The structure of product (G) is :
Text Solution
|
- The structure of product (H) is :
Text Solution
|
- The compound (B) is :
Text Solution
|
- The compounds ( C) and (D), respectively, are :
Text Solution
|
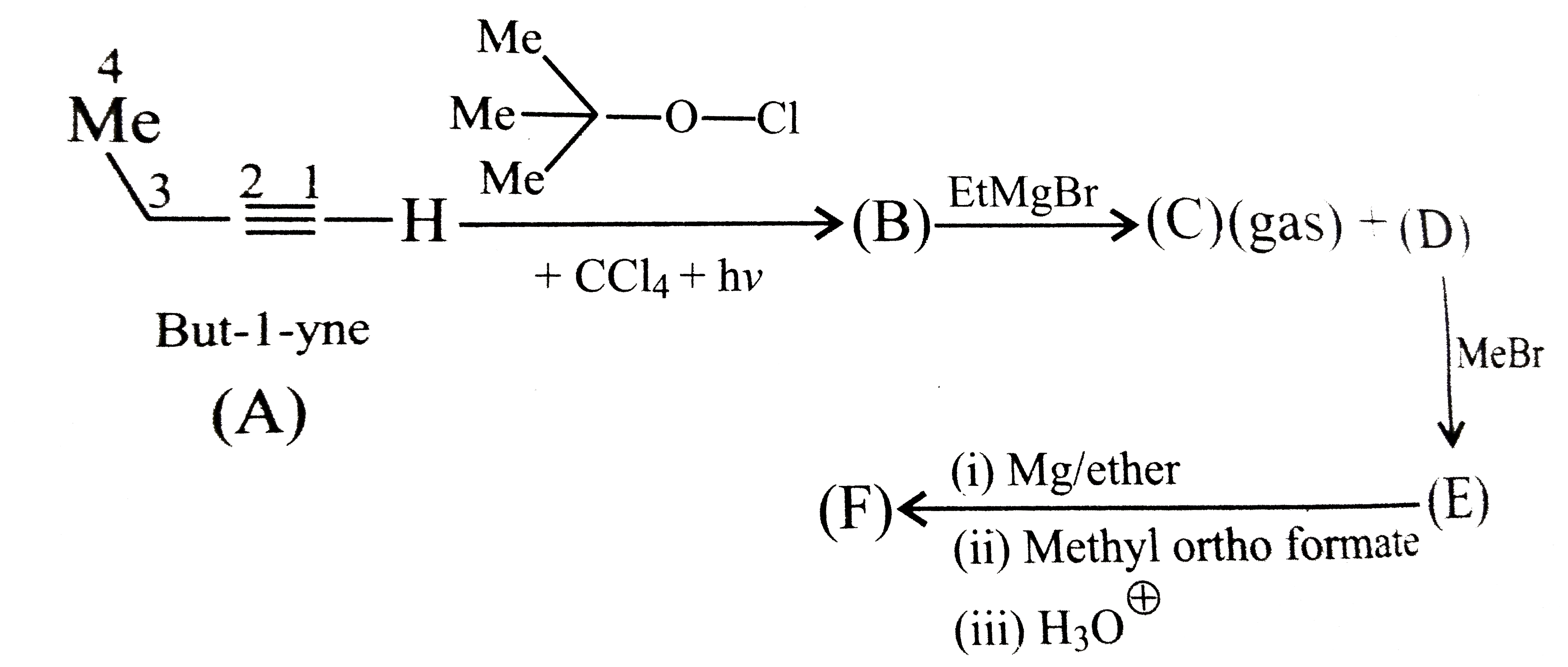
 .
.