A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|47 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Single Correct)|4 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|33 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Exercises (Multiple Correct)
- A 3^@ alcohol can be obtained by the reaction of PhMgBr and.
Text Solution
|
- A 3^@ alcohol can be obtained by the reaction of ketone (di-isopropyl...
Text Solution
|
- The coupling betweenC2 H5 MgBr and MeBr gives propane in the presence ...
Text Solution
|
- Which of the following halides does not form G.R when treated with mag...
Text Solution
|
- Which of the following halides does form G.R when treated with magnes...
Text Solution
|
- Acetophenone can be obtained by the reaction of PhMgBr and.
Text Solution
|
- Hexan-3-one can be obtained by the reaction of EtMgBr and.
Text Solution
|
- A 2^@ alcohol can be obtained by the reaction of di-t-butyl ketone an...
Text Solution
|
- . The compound (A) is :
Text Solution
|
- Which of the reagents is the most suitable for the following reaction ...
Text Solution
|
- Which of the following would give benzene when reacted with PhMgBr ?
Text Solution
|
- EtNH2 + MeMgI overset("Heated at high temp.") underset("in the presenc...
Text Solution
|
- Which of the following reactions would give pentan-2-ol ?
Text Solution
|
- Which of the following reactions would give caproic acid ?
Text Solution
|
- Which of the following statements is//are correct ?
Text Solution
|
- Which of the statements is/are correct ?
Text Solution
|
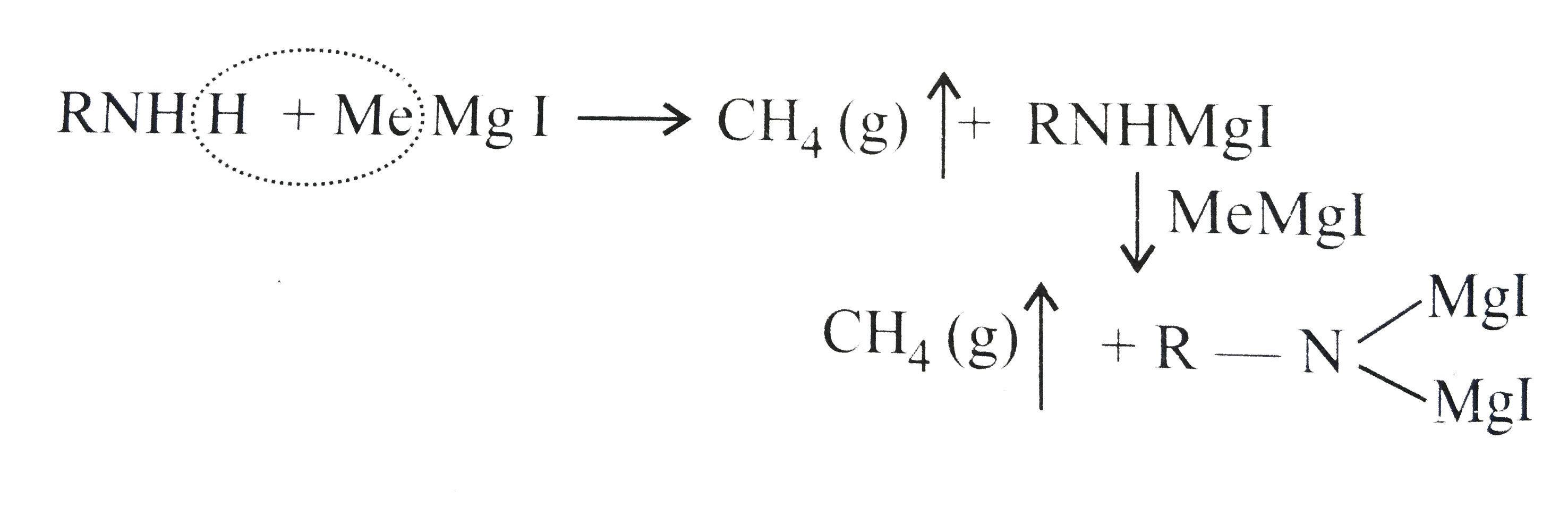 .
.