Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|34 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|35 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Solved Examples|17 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises (Subjective)
- Complete the following reactions : .
Text Solution
|
- Complete the following reactions : .
Text Solution
|
- Complete the following reaction : .
Text Solution
|
- Complete the following : (a) (b) ( c) (d) ( e)
Text Solution
|
- (a) Define the term neutralisation equivalent of an acid. (b) Find t...
Text Solution
|
- Neutralisation of 0.3504 gm of acid (A) requires 27.24 ml of 0.15 M Na...
Text Solution
|
- Which carboxylic acid (B) (N.E = 52 gm eq^-1) loses CO2 when heated to...
Text Solution
|
- Define saponification equivalent (S.E) of an ester. (b) When is S.E ...
Text Solution
|
- (a) Optically active compound (A) with a pleasant smell has a saponifi...
Text Solution
|
- The percentage of carbon, hydrogen, and nitrogen in an disibstituted a...
Text Solution
|
- An organic compound (A) composed of C, H, and O gives a characteristic...
Text Solution
|
- An acidic compound (A) (C4 H8 O3) loses its optical activity on strong...
Text Solution
|
- Write down the structures of the ( E) and (F). .
Text Solution
|
- Write down the structures of (G) and (H) where (G) is C4 H8 O3. .
Text Solution
|
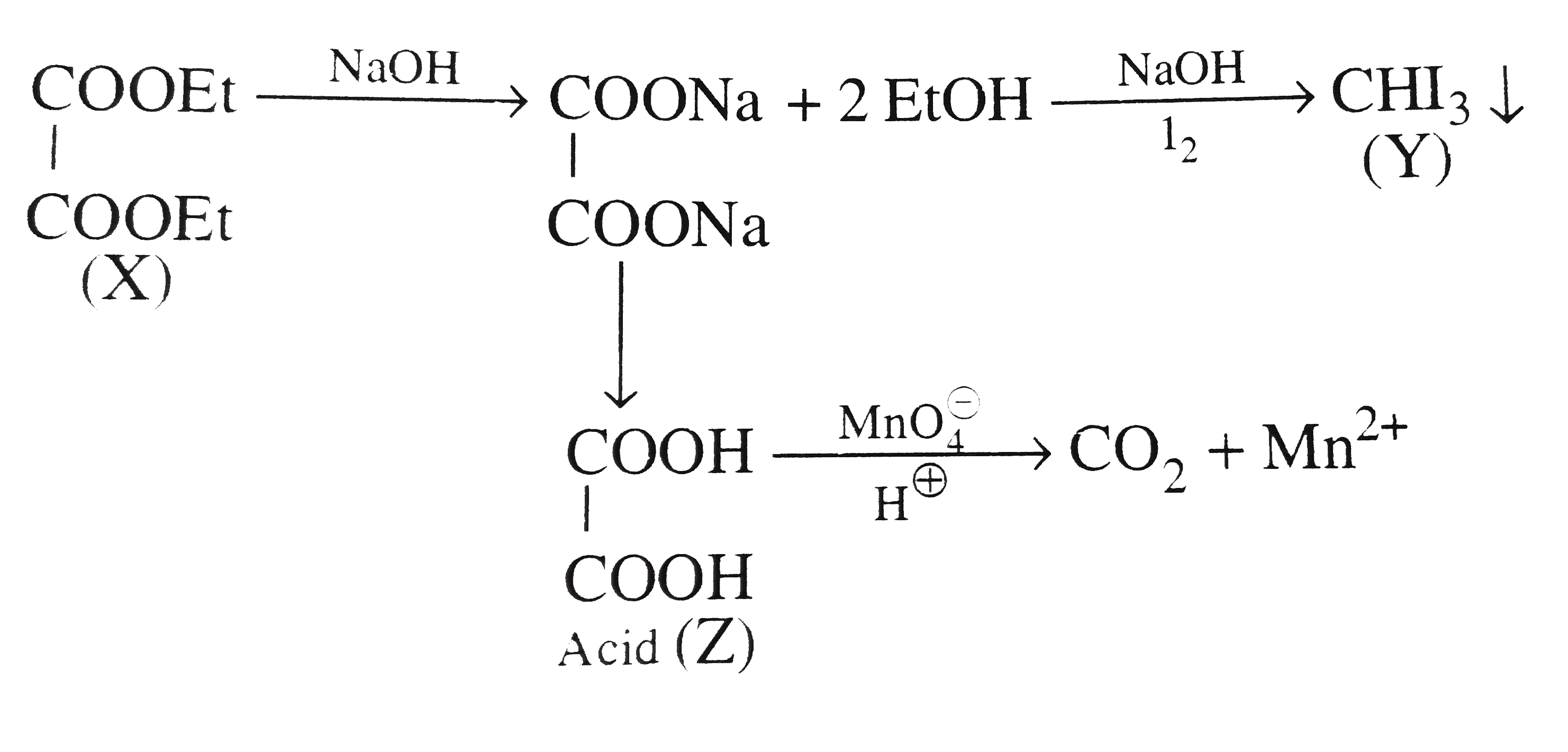
- A compound (X) with the molecular formula C6 H10 O4 is a neutral liqui...
Text Solution
|
- A dibasic organic acid gave the following results on analysis : 0.2496...
Text Solution
|
- An organic diabasic acid (A) gave the following on analysis : C = 57...
Text Solution
|
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- An organic compound (A) (C5 H8 O3) on heating with soda lime gives (B)...
Text Solution
|
 .
.