A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Single Correct)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Assertion-Reasoning)|2 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Singlecorrect)|64 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises (Assertion-Reasoning)
- The following reaction occurs. Acylium ion is resonance stabilise...
Text Solution
|
- AZT (azidothymine) is used to treat AIDS patients. If flights AIDS inf...
Text Solution
|
- (KOOC-CH=CH-COOK) undergoes Kolbe's reaction to form a product which r...
Text Solution
|
- 3-Phenol propanic acid (B) can be prepared by both nitrile and carbona...
Text Solution
|
- (I) is more acidic than The negative charge of the conjugate base of...
Text Solution
|
- Succinic acid (B) can be prepared by both nitrile and carbonation meth...
Text Solution
|
- beta-Hydroxy propanoic acid on heating gives acrylic acid. Acrylic a...
Text Solution
|
- Assertion: Both HCOOH (I) and CH3 COOH (II) give precipitate with aque...
Text Solution
|
- Acetic acid on reaction with hydrazoic acid (N3 H) in the presence of ...
Text Solution
|
- Oxides are more acidic than hydroxylamine (NH2 OH). Conjugate base ...
Text Solution
|
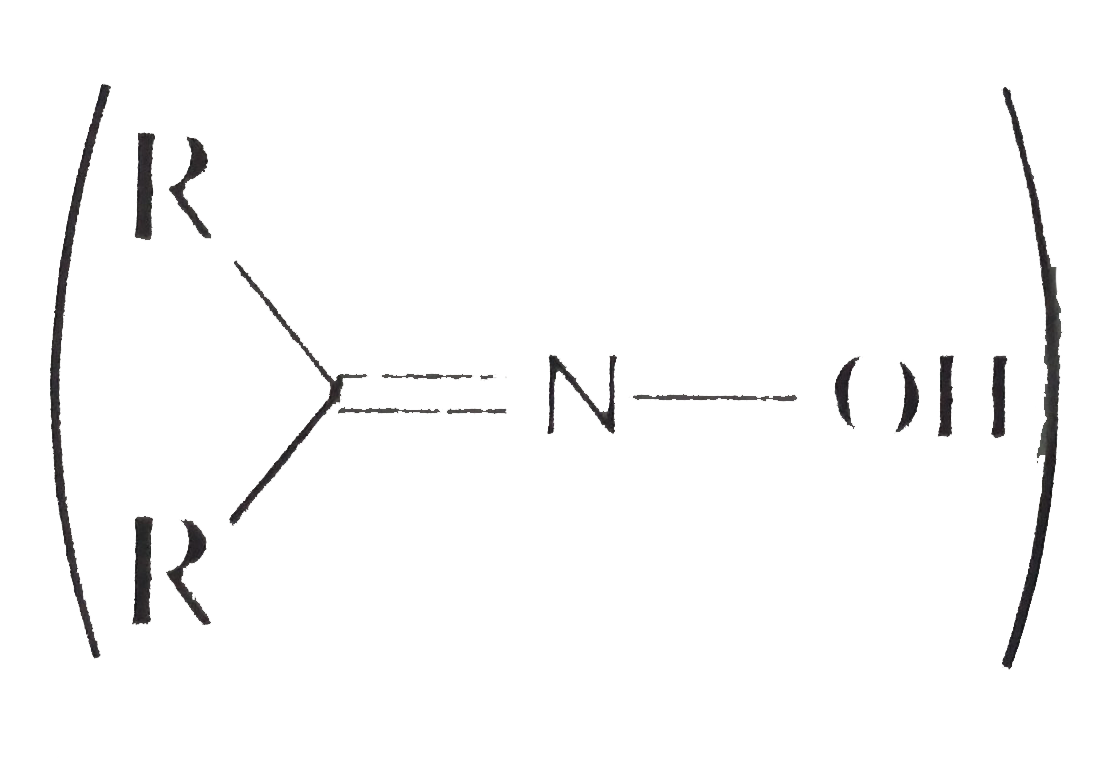 are more acidic than hydroxylamine `(NH_2 OH)`.
are more acidic than hydroxylamine `(NH_2 OH)`.