Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises Archives (Analytical And Descriptive)
- In the following, identify the compounds/reaction conditions represent...
Text Solution
|
- Arrange the following as stated : 'Increasing order of acidic streng...
Text Solution
|
- In the following identify the compounds/reaction conditions represente...
Text Solution
|
- Complete the following sequence of the reactions with approprite struc...
Text Solution
|
- Identify the major product in the following reactions : (i) (ii) ...
Text Solution
|
- In the following reactions, identify the compounds A, B, C and D. (i...
Text Solution
|
- Predict the major product in the following reaction : C(6) H(5)-CH(2...
Text Solution
|
- How will you bring about the following conversion ? 'Benzamide from ...
Text Solution
|
- Give reaction for the following : 'In acylium ion, the structure R-C ...
Text Solution
|
- Which of the following carboxylic acids undergoes decarboxylation easi...
Text Solution
|
- Complete the following sequence of reactions with appropriate structur...
Text Solution
|
- Complete the following giving the structures of the principal organic ...
Text Solution
|
- The following reaction gives two products. Write the structures of the...
Text Solution
|
- Explain briefly the formation of products giving the strcutures of the...
Text Solution
|
- What would be the major product in the following reactions ? .
Text Solution
|
- Write the structures of products (A) and (B) CH(3) -overset (O) over...
Text Solution
|
- Five isomeric para-disubsituted atomatic compounds (A) to ( E) with mo...
Text Solution
|
- A racemic mixture of (+-) 2-pheynl propanoic acid on esterification wi...
Text Solution
|
- A biologically active compound bombykol (C(16) H(30)O) is obtained fro...
Text Solution
|
- Convert the following (in not more than three steps): .
Text Solution
|
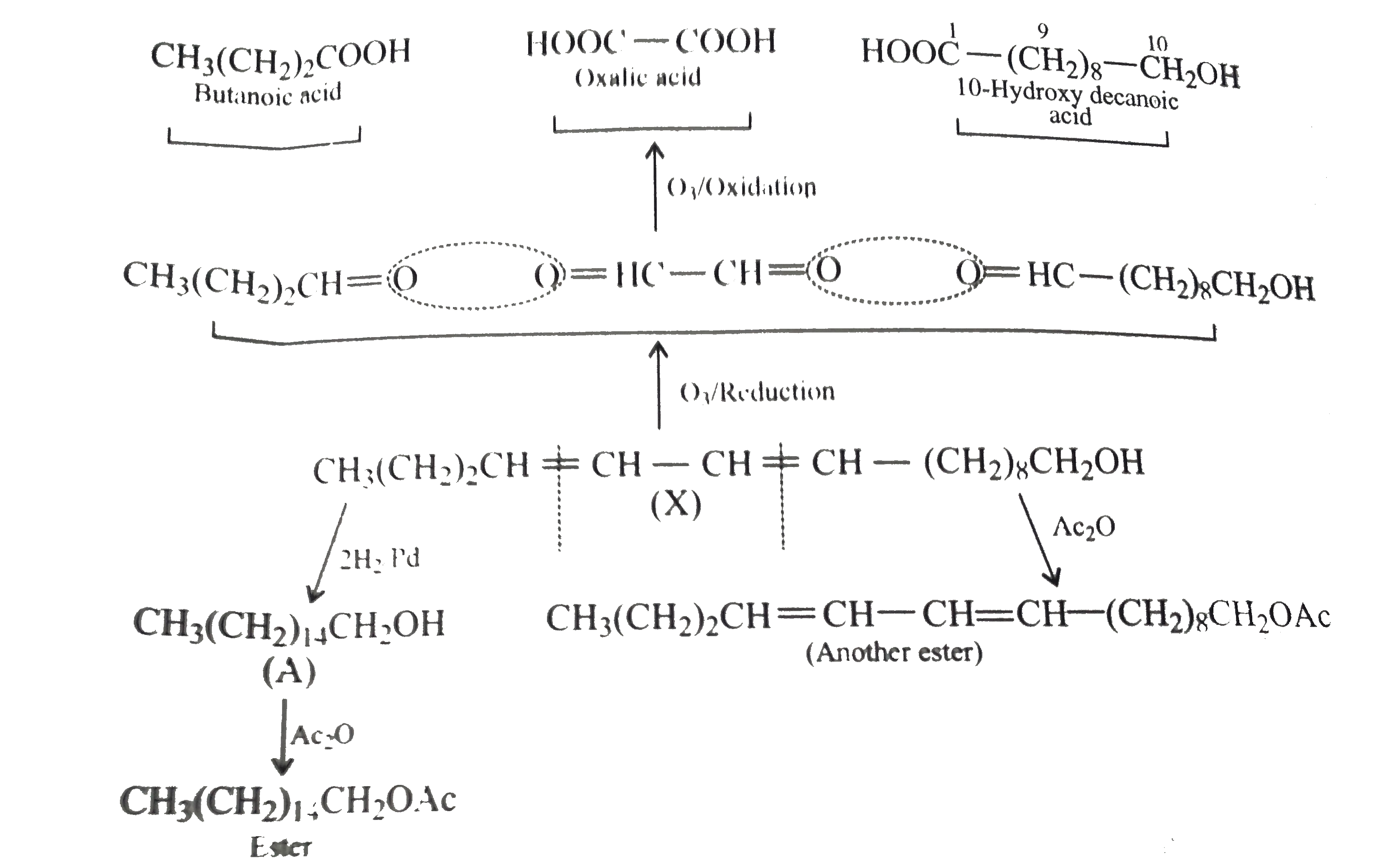 .
.