Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Exercises Subjective|21 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Exercises Concept Application|13 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Archives Analytical And Descriptive|24 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Solved Examples
- Distinguish between the following pairs: {:((I),,.(II)),(a.(PhNH(3))...
Text Solution
|
- Complete the following reactions. a. PhNO(2) overset(Zn+aq.NH(4)CI)r...
Text Solution
|
- Give the reagents in the following reactions:
Text Solution
|
- Complete the following reactions a. underset((A))(PhNH(2)) overset(H...
Text Solution
|
- Explain the formation of the mixture PhCH(2)CHO (I) and PhCOMe (II) wh...
Text Solution
|
- a. Distinguish between 1^(@),2^(@), and 3^(@) amines by using succine ...
Text Solution
|
- Identify compounds (A) through (E) in the following: p-NO(2)C(6)H(4)...
Text Solution
|
- Identify compound (A) to (E) in the following:
Text Solution
|
- (a) Identify the products: (b) Identify (A),(B),(X),(Y) and (Z) i...
Text Solution
|
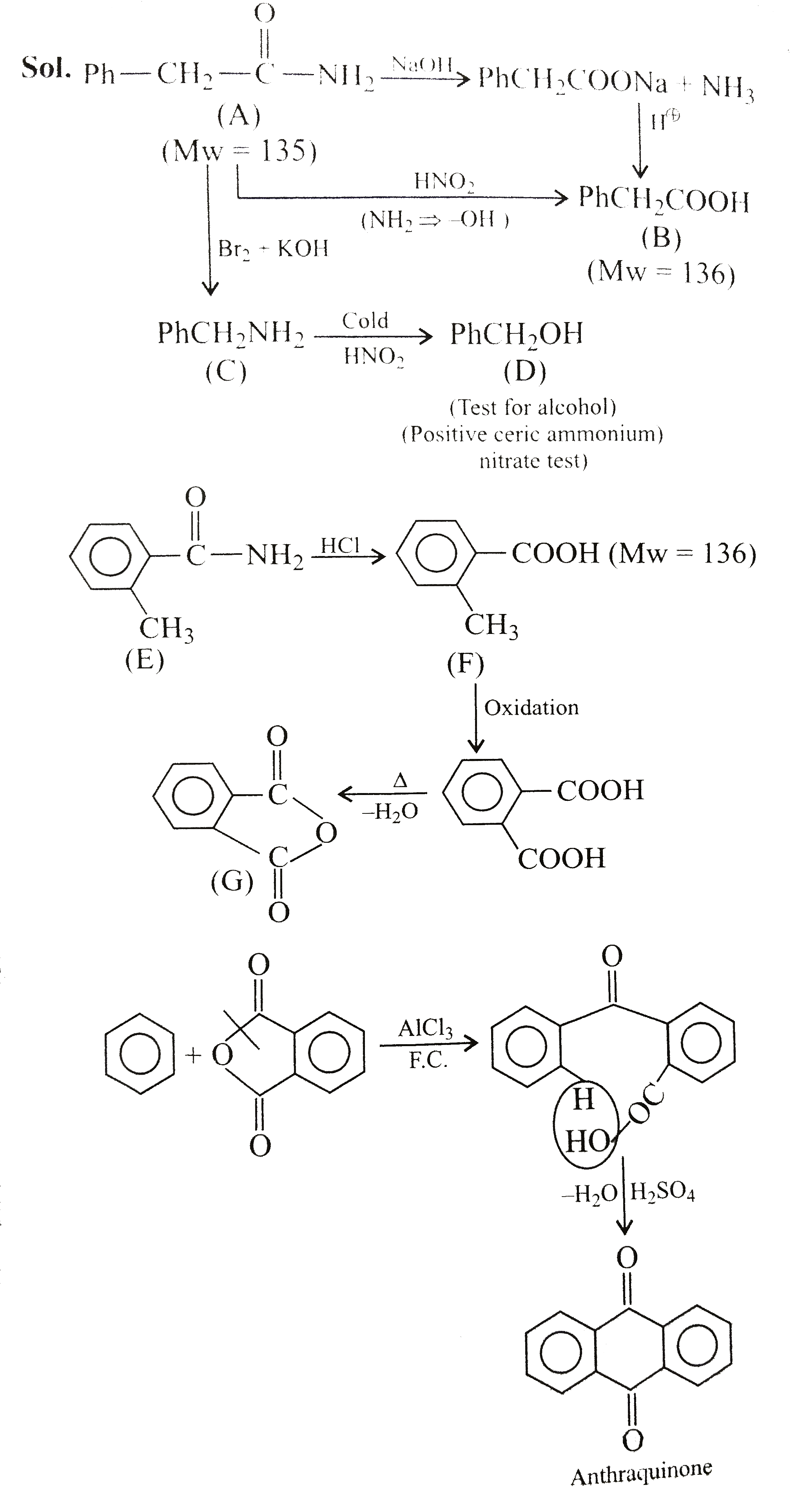
- An organic compound (A) of molecular. Weight 135 on boiling with NaOH ...
Text Solution
|
- The aqueous solution of a nitrogen and chlorine containing organic com...
Text Solution
|
- A mixture of two organic compound is added to cold water. After filtra...
Text Solution
|