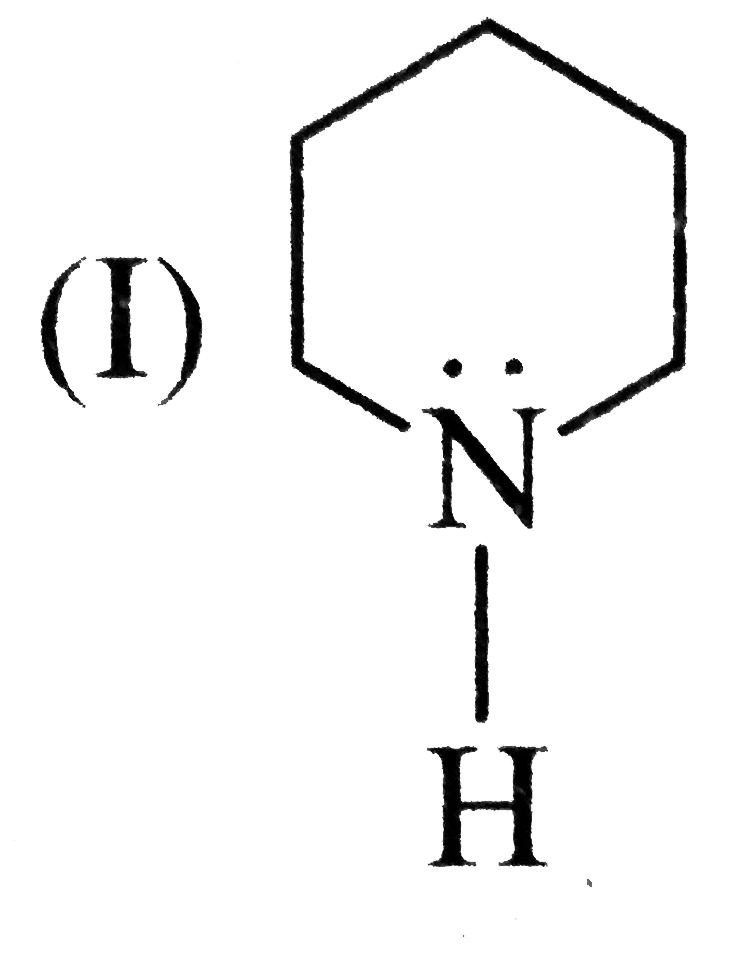
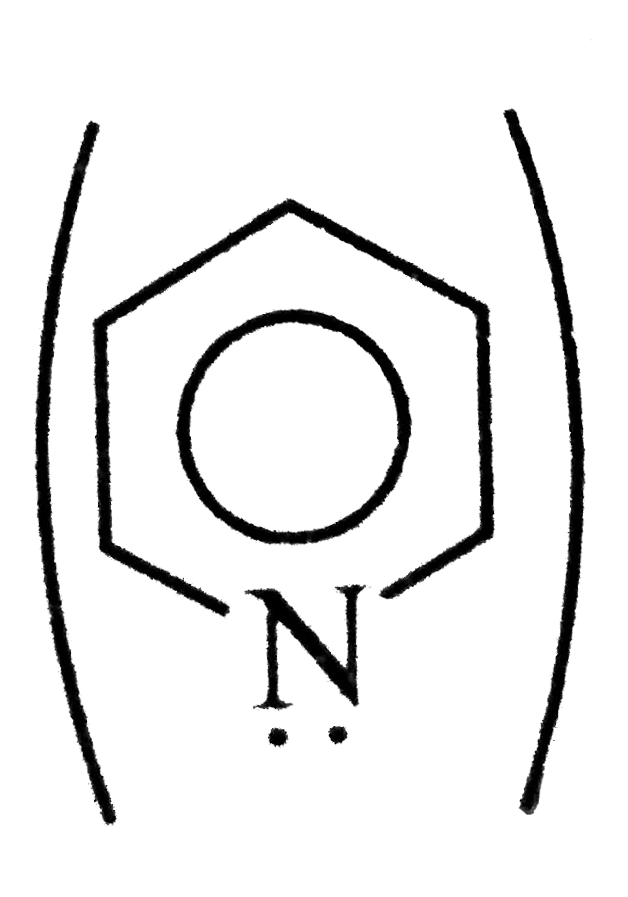
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Archives Single Correct|10 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Archives Assertion-Reasoning|2 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|54 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|13 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP-Exercises Assertion And Reasoning
- Assertion(A) : Aniline hydrogen sulphate on heating froms a mixture of...
Text Solution
|
- Assertion (A) : Ph overset (o+)(N2) Br^(Θ) couples with N, N-dimethyl...
Text Solution
|
- Assertion (A) : Gabriel phthalimide reaction is used for the preparti...
Text Solution
|
- Assertion (A) : Pyridine is more basic than piperidine. Reason (R ):...
Text Solution
|
- Assertion (A) : Ph overset (o+) (N2) Br^(Θ) is more acidic than NH4 B...
Text Solution
|
- Assertion (A) : Carbylamine reaction takes place between 1^@ amine and...
Text Solution
|
- Assertion (A) : Hofmann bromamide reaction takes place btween an arth...
Text Solution
|
- Assettio (A) : Ph overset (o+) (N2) Br^(Θ) on reaction with NaOH gives...
Text Solution
|
- Assettion (A) : Ph overset (o+) (N2) Br^(Θ) pm reactopm wotj motrpnem...
Text Solution
|
- Assettion (A) : Coupling of Ph overset (o+) (N2) Br^(Θ) with aniline i...
Text Solution
|