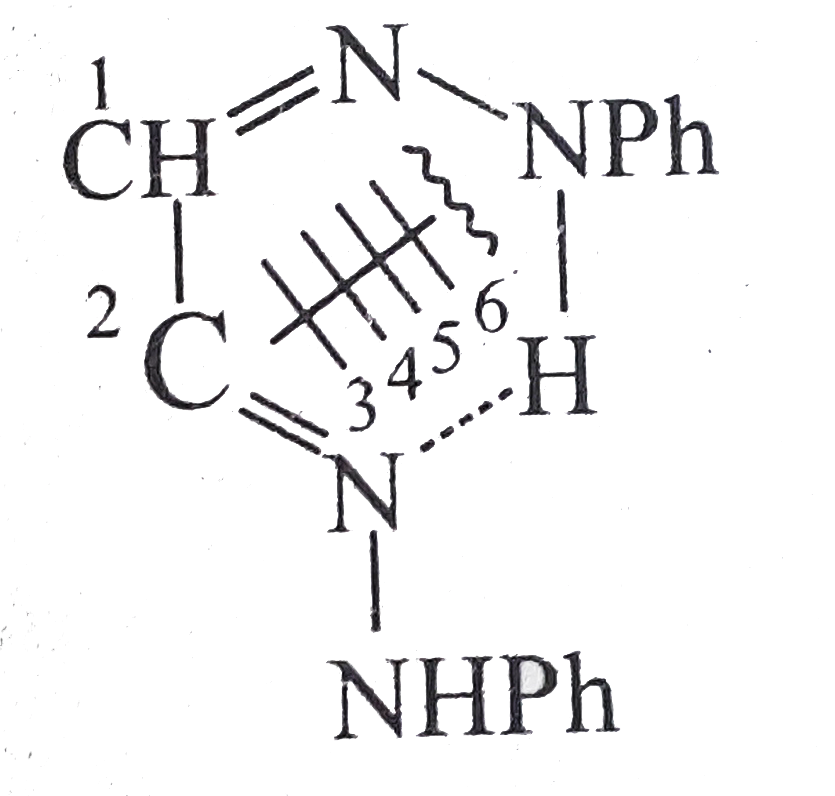
Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
CENGAGE CHEMISTRY|Exercise Solved Examples|45 VideosBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Concept Application)|25 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-BIOMOLECULES-Exercises Archives (Analytical And Descriptive)
- (a). Glucose and fructose form the same osazone, what does it prove? ...
Text Solution
|
- Write the structure of alanine at pH=2 and pH=10.
Text Solution
|
- Give the structure of each of the products in the following reactions:...
Text Solution
|
- As partune, an artifical sweetener, is a peptide and has the following...
Text Solution
|
- Write down the heteroheneous catalyst involved in the polymerisation o...
Text Solution
|
- Following two amino acids liosine and glutamine form dipeptide linkage...
Text Solution
|
- The structure of D-glucose is as follows: (a) Draw the structure ...
Text Solution
|
- Which of the following disaccharides will not reduce Tollens reagent?
Text Solution
|
- Arrange in the order of increasing acidic strengths.
Text Solution
|