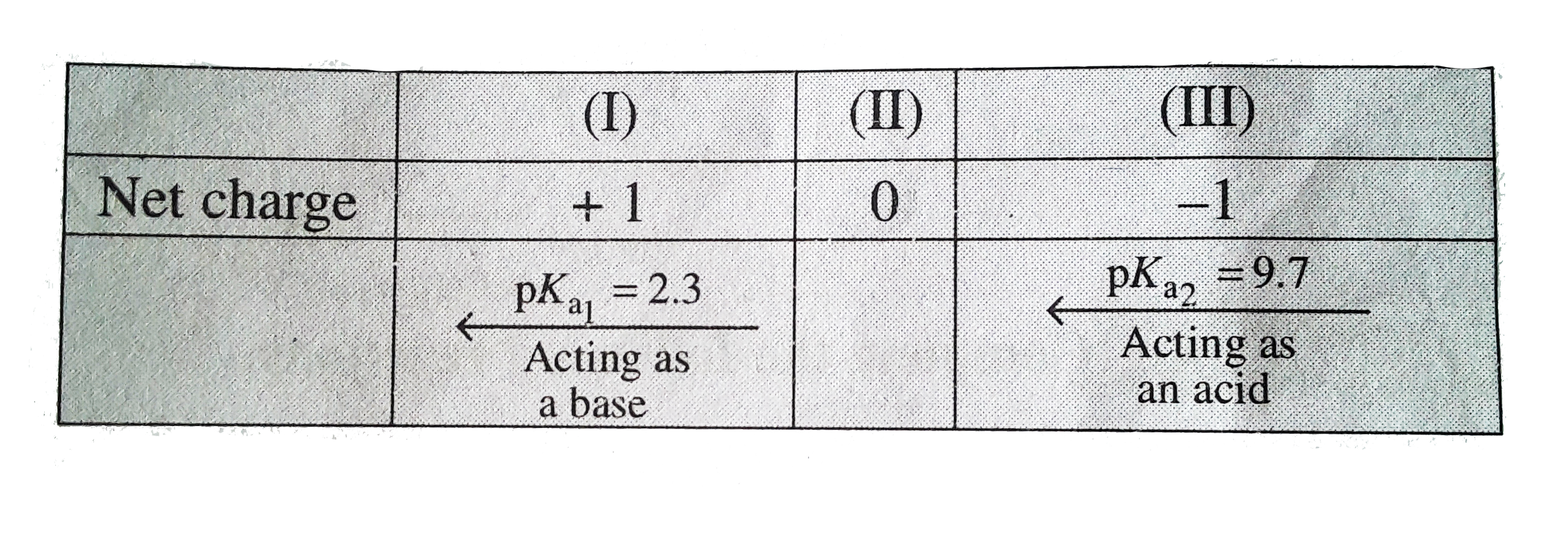Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Concept Application)|25 VideosBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|38 VideosBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-BIOMOLECULES-Solved Examples
- Give the structure and IUPAC name of disaccharide, which gives the rea...
Text Solution
|
- a. Give a chemical test of system with I(2). b. What is the change i...
Text Solution
|
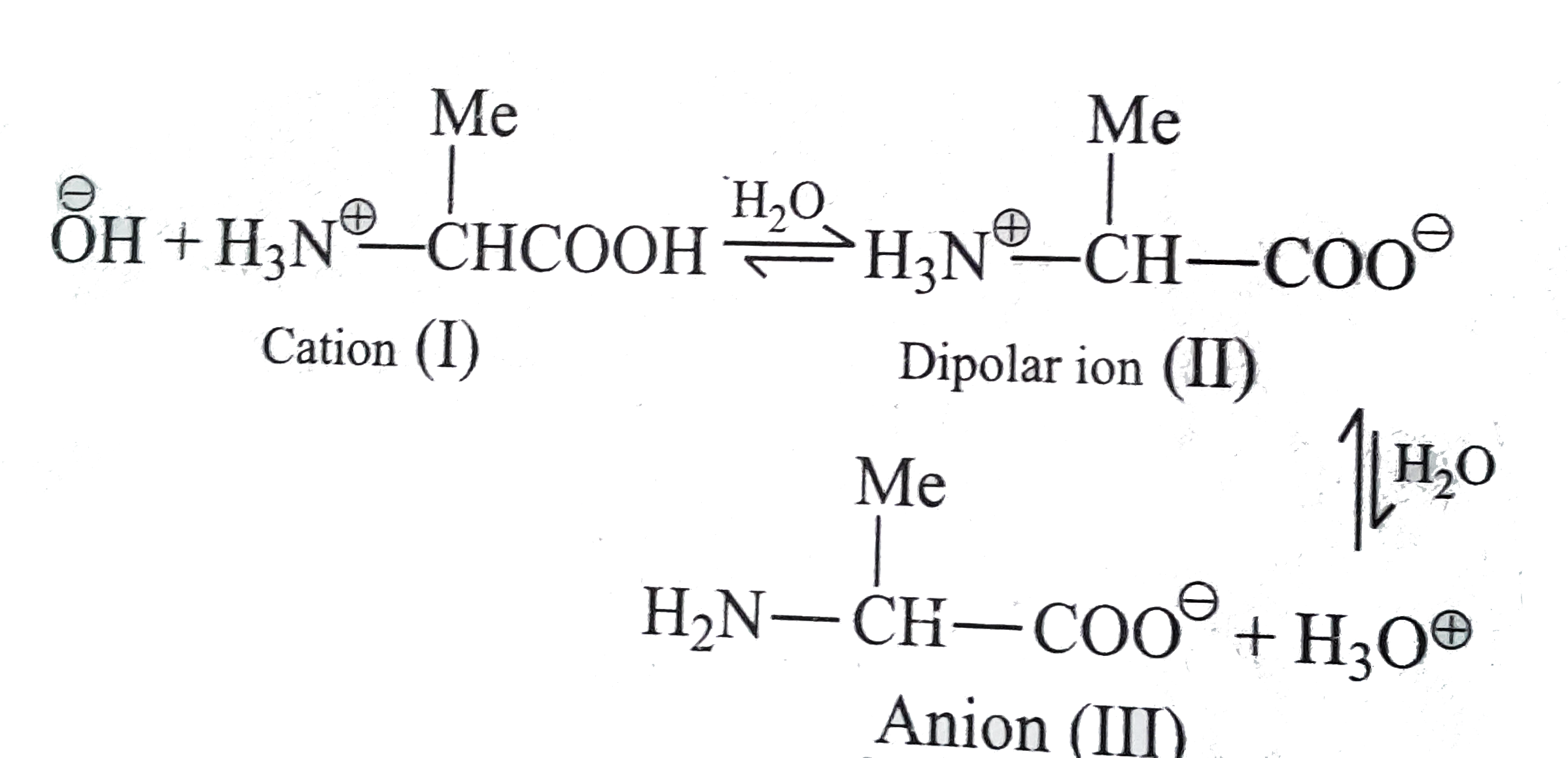
- a. Write the equilibrium reaction to show the amphoteric behaviour of ...
Text Solution
|
- a. How is the mixture of aspartic acid (A) histidine (B) and threonin...
Text Solution
|
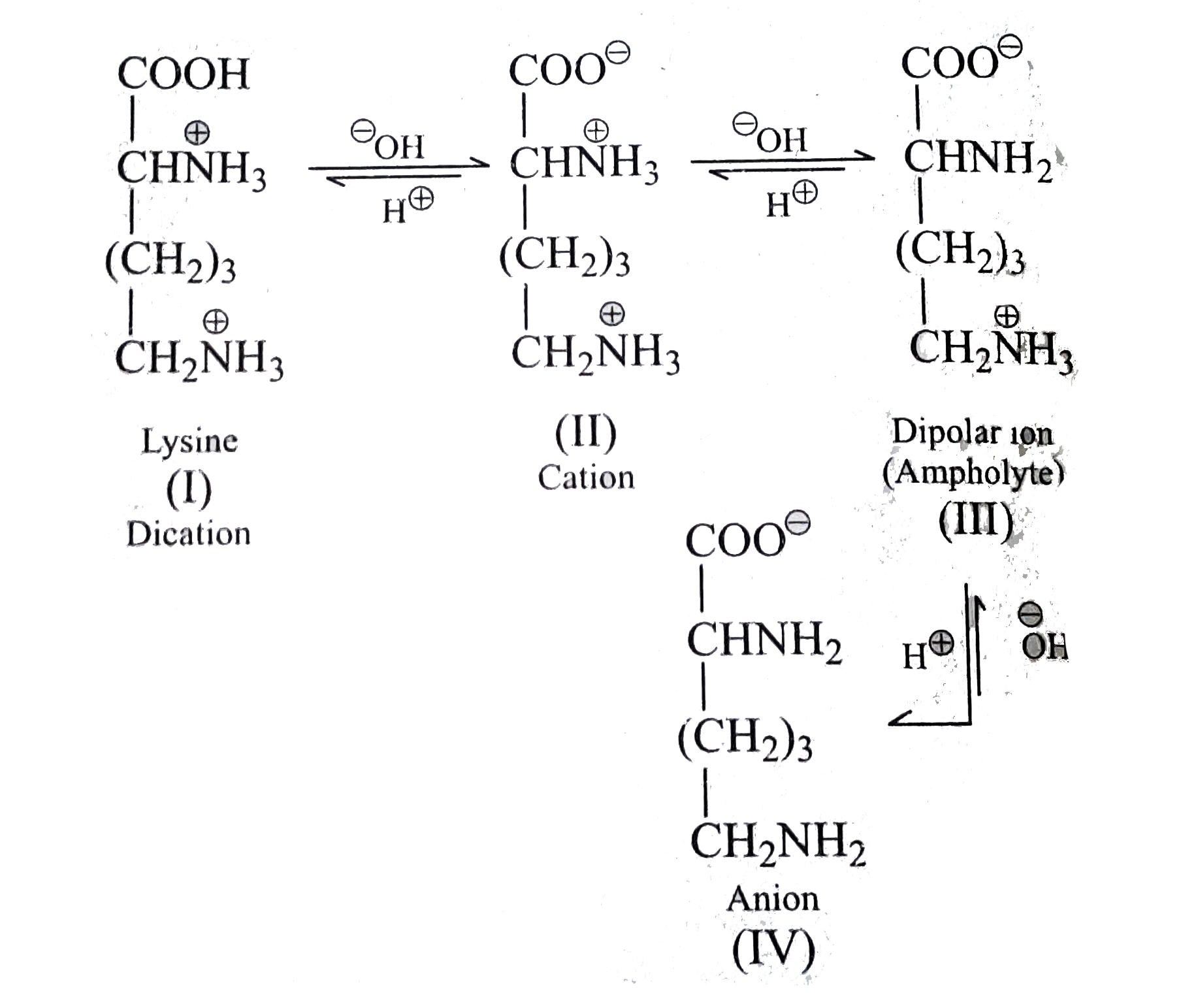
- a. Write the structure of histidine when pHlt1.82 and pHgt1.82. b. ...
Text Solution
|
- Define the terms: a. Gene , b. Genetic code, c. Transcription, d...
Text Solution
|
- List three functions of nucleotides in a cell.
Text Solution
|
- The two strands of DNA are not identical, but are complementary'. Expl...
Text Solution
|
- Write two functions of carbohydrates in plants.
Text Solution
|
- What type of bonding occurs in globular protein?
Text Solution
|
- What will be the sequence of bases on mRNA molecule synthesised on the...
Text Solution
|
- Name one reducing and one nonreducing disaccharide.
Text Solution
|
- Name on fibrous and one globular protein.
Text Solution
|
- What causes sickle cell anaemia?
Text Solution
|
- Give one example of a denatured protein.
Text Solution
|
- Name a polypeptide hormone which maintains the glucose level in the bl...
Text Solution
|
- What is nucleoside?
Text Solution
|
- What is nucleoside?
Text Solution
|
- Which purine and pyrimidine bases are present in DNA and RNA?
Text Solution
|
- What are waxes?
Text Solution
|