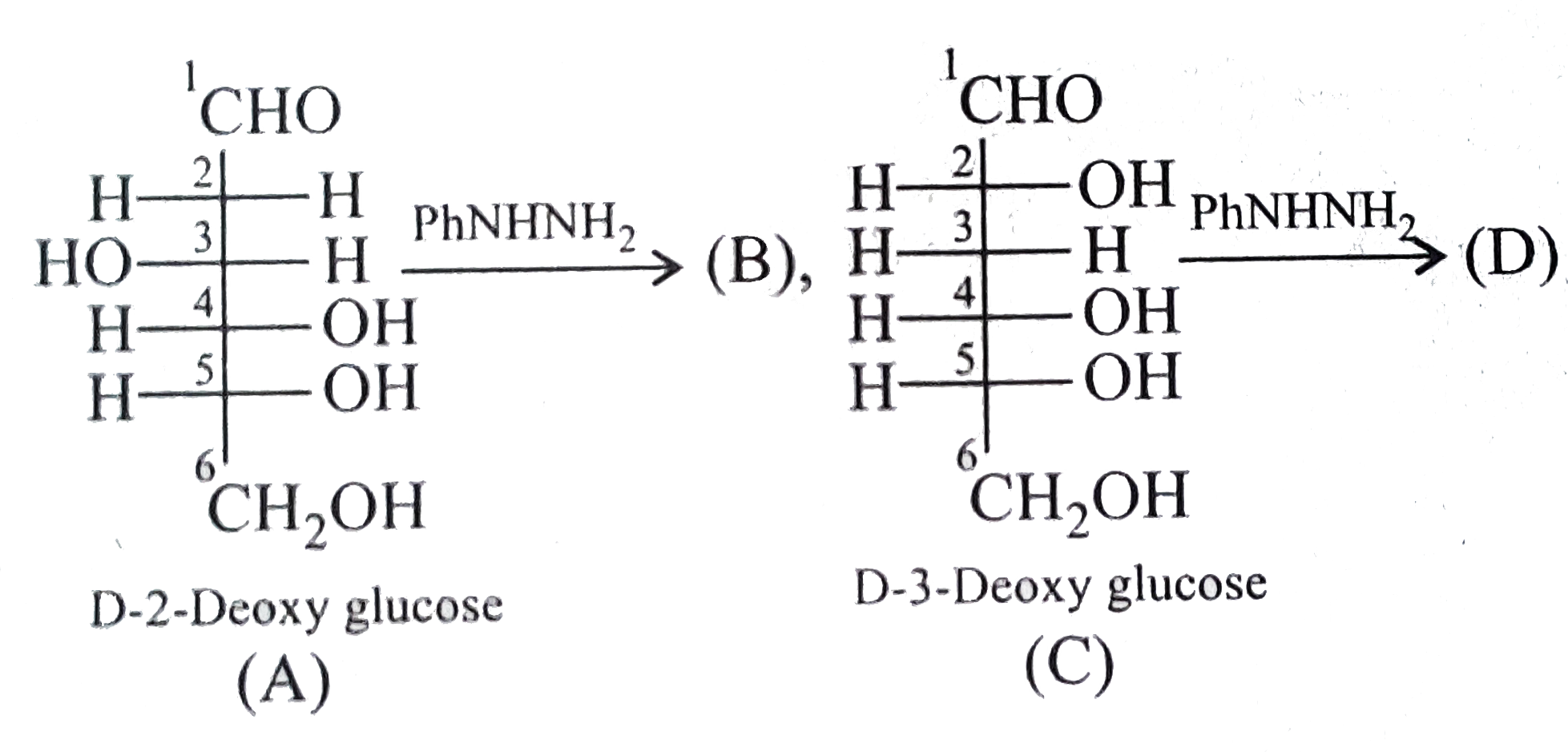
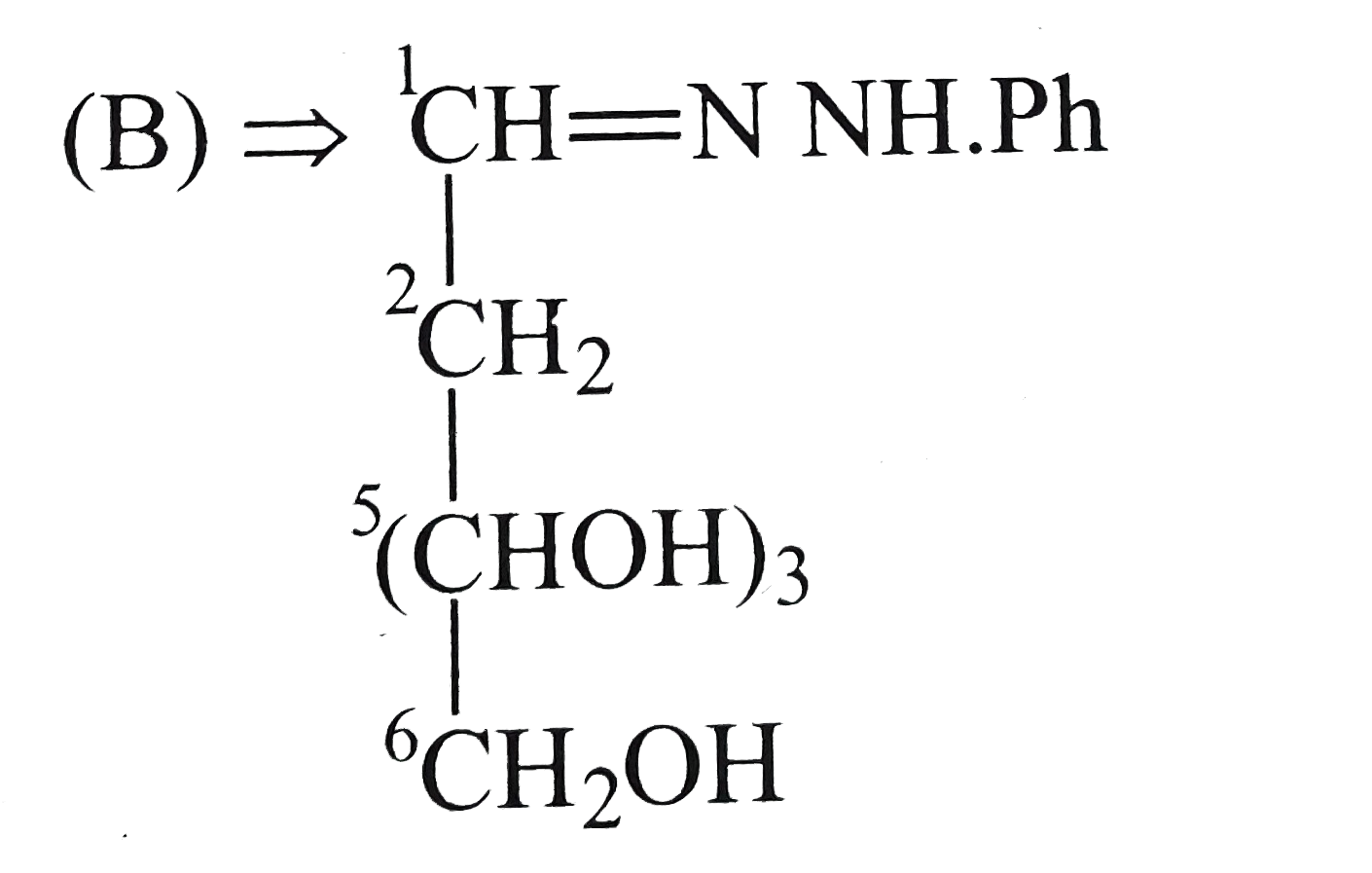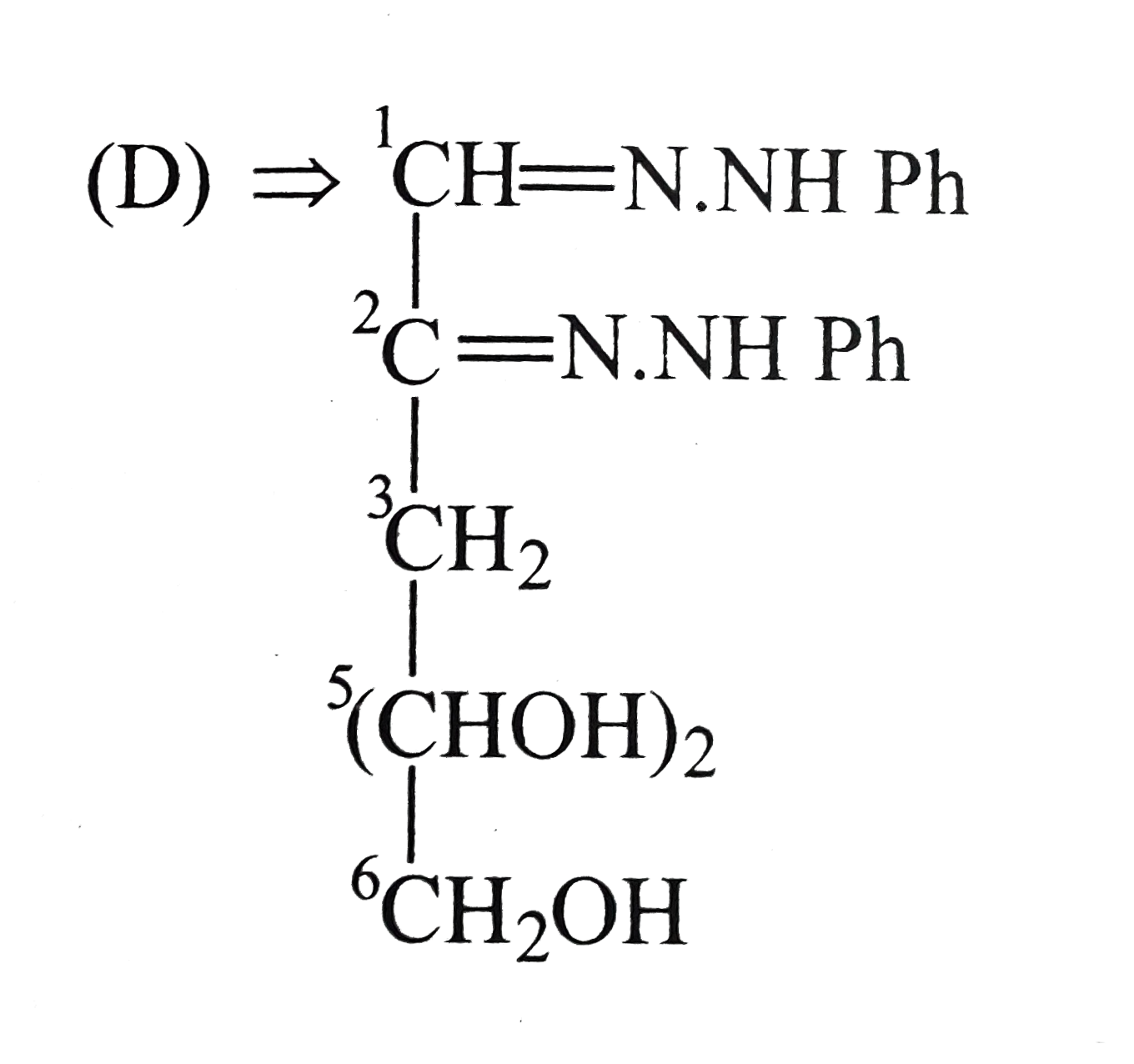A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correcttype)|31 VideosBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Single Correcttype)|81 VideosBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises (Concept Application)|25 VideosAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-BIOMOLECULES-Exercises (Linked Comprehension)
- Compound (B) is:
Text Solution
|
- How many moles of PhNHNH(2) react with 1 mol (A)?
Text Solution
|
- The compound (D) is:
Text Solution
|
- How manty moles of (C ) react with 1 mol PhNHNH(2)
Text Solution
|
- The dipeptide (III) is:
Text Solution
|
- The tripeptide (II) is:
Text Solution
|
- The tetrapeptide (I) is:
Text Solution
|
- Compound 'A' has many functional groups.
Text Solution
|
- Compound (B) is:
Text Solution
|
- Compound (C ) is:
Text Solution
|
- Compound (D) is:
Text Solution
|
- Account for the acidity of L-ascorbic acid (pK(a)=4.21). Which of the ...
Text Solution
|
- How many chrial centres are in (G) (L-ascorbic acid) ?
Text Solution
|
- Aldohexose reacts with acetone in H(2)SO(4) to form cyclic ketal. Nece...
Text Solution
|
- The structure of (G) is shown below: Which of the statement(s) i...
Text Solution
|
- If the lacetone formation in compound (G) takes place between C-1 and ...
Text Solution
|
- Two isomeric products are obtained in (B). They are:
Text Solution
|
- The sequence from (A) to (D+E) is called:
Text Solution
|
- Two isomeric products are obtained in (C ) they are:
Text Solution
|
- The compounds (F) and (G), respectively, are:
Text Solution
|