A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET MAJOR TEST (COACHING)-MAJOR TEST 6-PHYSICS
- A mass of 1kg is suspended by a string A. Another string C is connecte...
Text Solution
|
- A body is tied up a string of length l and rotated in vertical circle ...
Text Solution
|
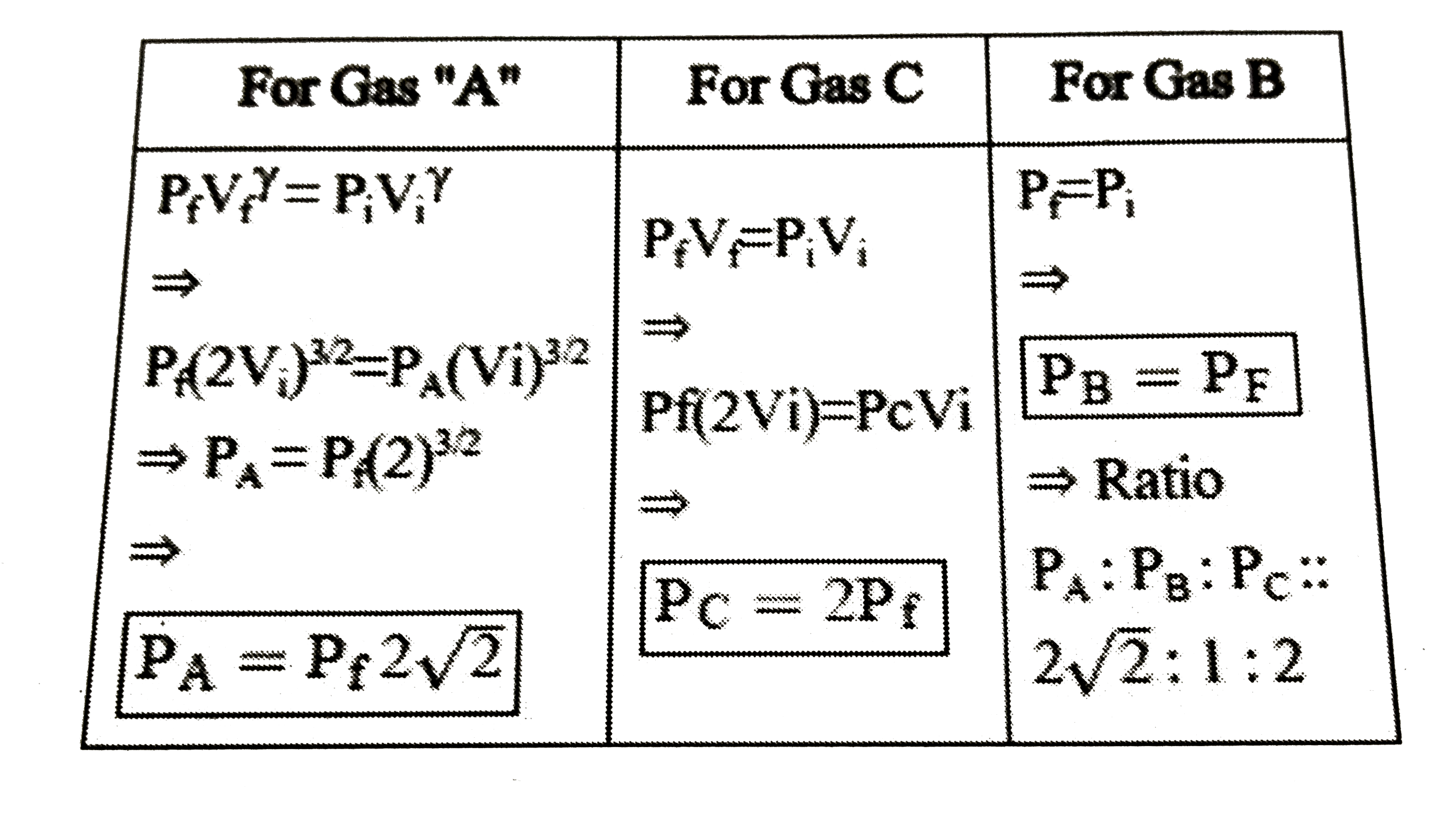
- Three samples of the same gas A,B and C (gamma=3//2) have initially eq...
Text Solution
|
- if the velocity of sound in helium at room temperature is 330 m/s, the...
Text Solution
|
- To get an output 1 from the circuit shown in the figure, the input mus...
Text Solution
|
- In the nuclear raction .1H^2 +.1H^2 rarr .2He^3 +.0n^1 if the mass of ...
Text Solution
|
- The first operation involved in a Carnot cycle is
Text Solution
|
- The amplitude of damped oscillator decreased to 0.9 times its origina...
Text Solution
|
- Two coherent sources have intensity ratio of 100 : 1, and are used for...
Text Solution
|
- In an electromagnetic wave, the amplitude of magnetic field is 3xx10^(...
Text Solution
|
- The correct formula for potential energy in the electric field of an e...
Text Solution
|
- The rotation of the earth about its axis speeds up such that a man on ...
Text Solution
|
- 50 gram of ice at 0^(@)C is mixed with 50 gram of water at 60^(@)C , f...
Text Solution
|
- Find current through Zener diode–
Text Solution
|
- The half-life period of a radioactive substance is 5 min. The amount o...
Text Solution
|
- Two radiation of photons energy 3eV and 4.5 eV successively illuminate...
Text Solution
|
- The coefficient of linear expansion of crystal in one direction is alp...
Text Solution
|
- When radiation of wavelength lambda is incident on a metallic surface...
Text Solution
|
- When a plane wave front incident on a concave mirror as shown in figur...
Text Solution
|
- Eight equal water drops are falling in air with a steady velocity 4cms...
Text Solution
|