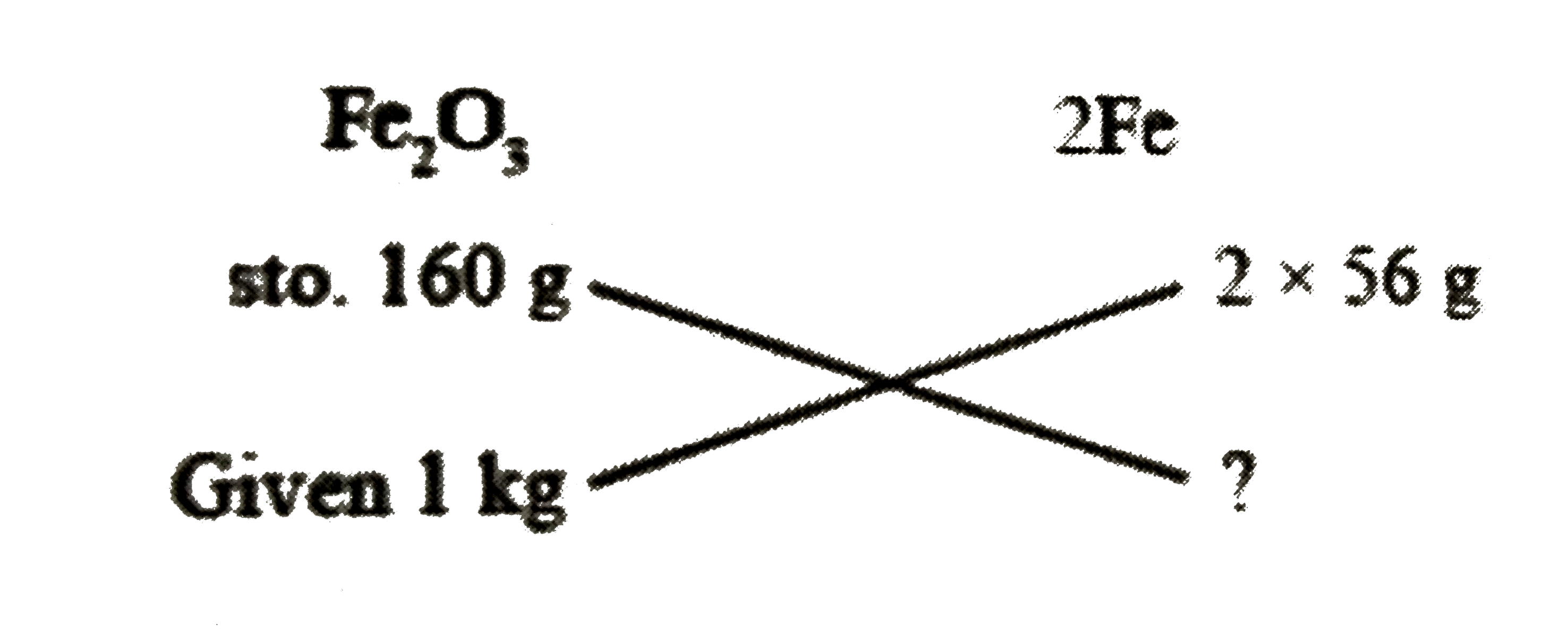
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Fe(2)O(3) + 3H(2) to 3H(2)O + 2Fe Calculate the weight of Fe obtaine...
Text Solution
|
- Calculate DeltaH at 85^(@)C for the reaction: Fe(2)O(3)(s) +3H(2)(g)...
Text Solution
|
- Calculate standard entropy change in the reaction Fe(2)O(3)(s)+3H(2)(g...
Text Solution
|
- Fe(2)O(3)(s) may be converted to Fe by the reaction Fe(2)O(3)(s)+3H(...
Text Solution
|
- The reactions which is not involved in the extraction of iron from the...
Text Solution
|
- For the reaction Fe(2)O(3)+3CO rarr 2Fe+3CO(2) , the volume of carbon ...
Text Solution
|
- Fe(2)O(3) + 3H(2) to 3H(2)O + 2Fe Calculate the weight of Fe obtained ...
Text Solution
|
- The DeltaH at 358 K for the reaction Fe(2)O(3)(g)+3H(2)(g) to 2Fe(s)+3...
Text Solution
|
- The reaction between aluminium and ferric oxide can generate temperatu...
Text Solution
|