Similar Questions
Explore conceptually related problems
Recommended Questions
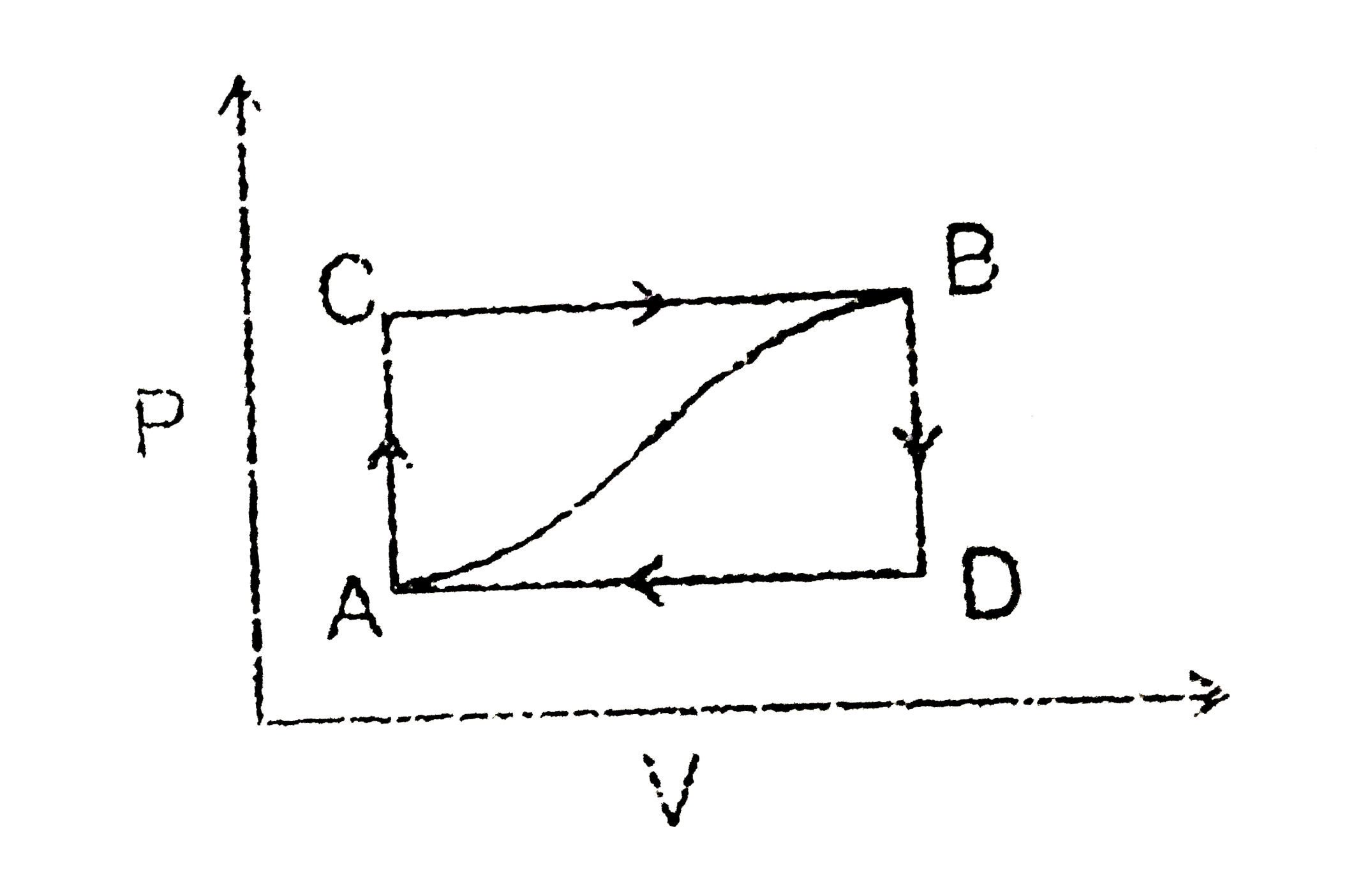
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- A system is taken from state a to state c along the path adc (figure)....
Text Solution
|
- How much heat flows into the system along path ADB it the work done by...
Text Solution
|
- When the system is retuned form state B to A along the curved path, th...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- When a system is taken from state A and B along the path ACB, 80J of h...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|