Similar Questions
Explore conceptually related problems
Recommended Questions
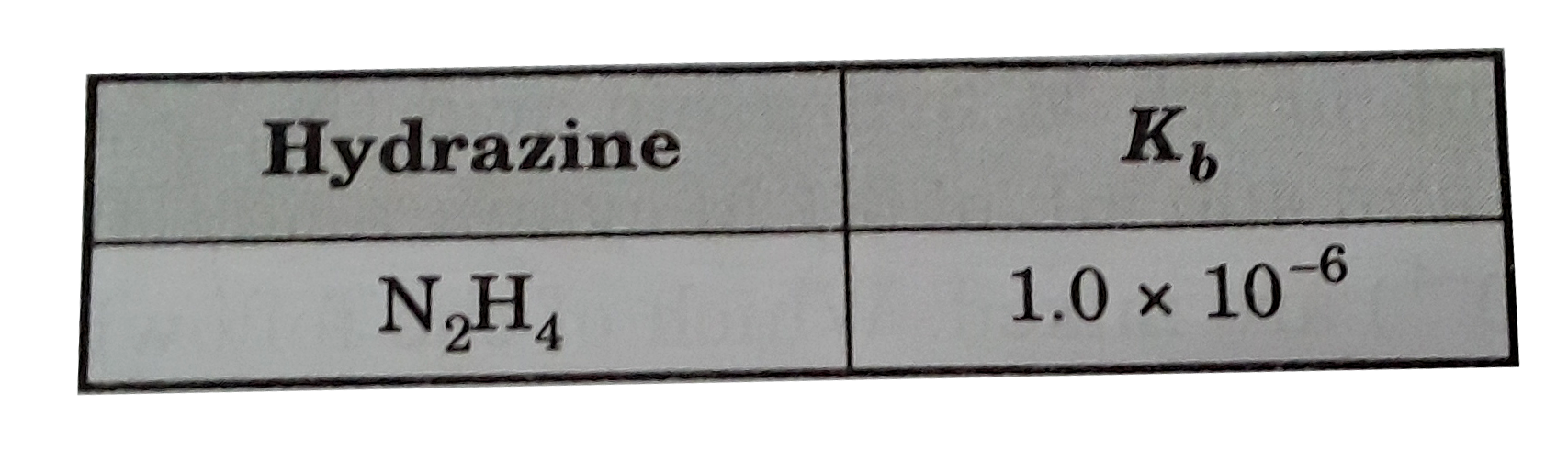
- What is the pH of a 0.15 M solution of hydrazine , N2H4?
Text Solution
|
- In hydrazine (N2H4), nitrogen ishybridized.
Text Solution
|
- What is the pH of a 0.15 M solution of formic acid, HCOOH ? {:("Formic...
Text Solution
|
- Calculate the pH of a 0.15 M solution of HOCl.
Text Solution
|
- What is the pH of a 0.15 M solution of hydrazine , N2H4?
Text Solution
|
- 100 mL of 0.15 M HCl is mixed with 100 mL of 0.005M HCl , what is the ...
Text Solution
|
- In N2H4 (hydrazine) both the nitrogen atoms are
Text Solution
|
- The pH of a solution obtained by mixing 60 mL of 0.1 M NaOH solution a...
Text Solution
|
- The pH of 0.04 M hydrazine solution is 9.7 . Calculate its ionization ...
Text Solution
|