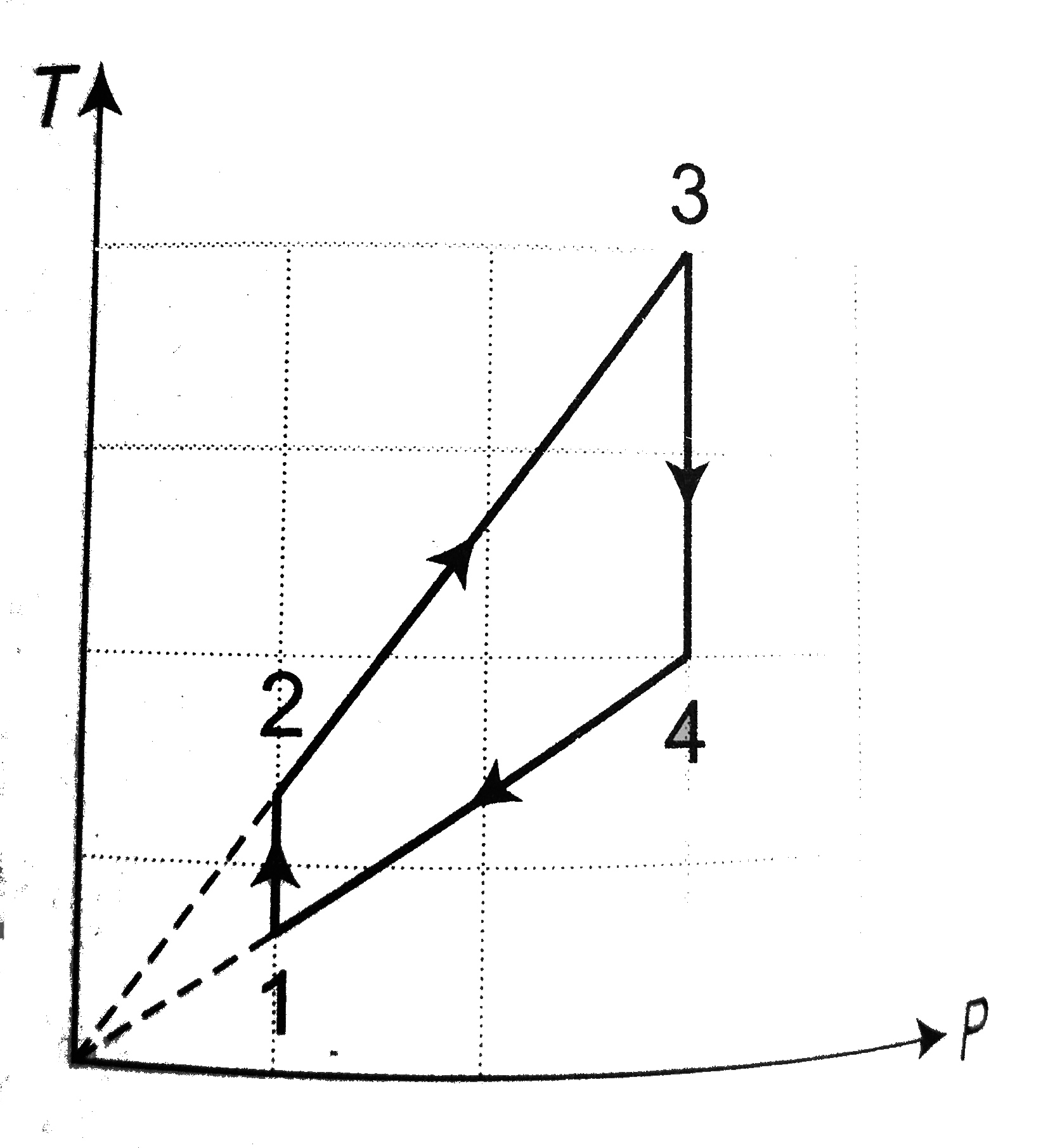
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Second Law Of Thermodynamics|29 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Problems Based On Mixed Concepts|49 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise First Law Of Thermodynamics , Internal Energy And Work Done|55 VideosGRAVITATION
A2Z|Exercise Chapter Test|29 VideosMOCK TEST
A2Z|Exercise Motion With Constant Acceleration|15 Videos
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-Application Of First Law Of Thermodynamics In Different Situations
- For one complete cycle of a thermodynamic process gas as shown in the ...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic process abca . It...
Text Solution
|
- A thermodynamic process of one mole ideal monoatomic gas is shown in f...
Text Solution
|
- A gas is expanded form volume V(0) to 2V(0) under three different pro...
Text Solution
|
- During adiabatic process pressure P versus density roh equation is
Text Solution
|
- P-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- An ideal gas is taken from state 1 to state 2 through optional path A,...
Text Solution
|
- In the following P-V diagram two adiabatics cut two isothermals at tem...
Text Solution
|
- In an adiabatic process, R = (2)/(3) C(v). The pressure of the gas wil...
Text Solution
|
- Initial volume of three samples of same gas A, B and C are same. The p...
Text Solution
|
- One mole of an ideal gas at temperature T expands slowly according to ...
Text Solution
|
- If the ratio of specific heat of a gas of constant pressure to that at...
Text Solution
|
- A polytropic process for an ideal gas is represented by equation PV^(n...
Text Solution
|
- During an experiment, an ideal gas is found to obey an additional law ...
Text Solution
|
- Find the amount of work done to increase the temperature of one mole o...
Text Solution
|
- Two moles of an ideal mono-atomic gas undergo a cyclic process as show...
Text Solution
|
- A sample of an ideal gas initially having internal energy U(1) is allo...
Text Solution
|
- An amount Q of heat is added to a monoatomic ideal gas in a process in...
Text Solution
|
- In a process, the molar heat capacity of a diatomic gas is (10)/(3) R....
Text Solution
|
- P-V graph for an ideal gas undergoing polytropic process PV^(m) = cons...
Text Solution
|