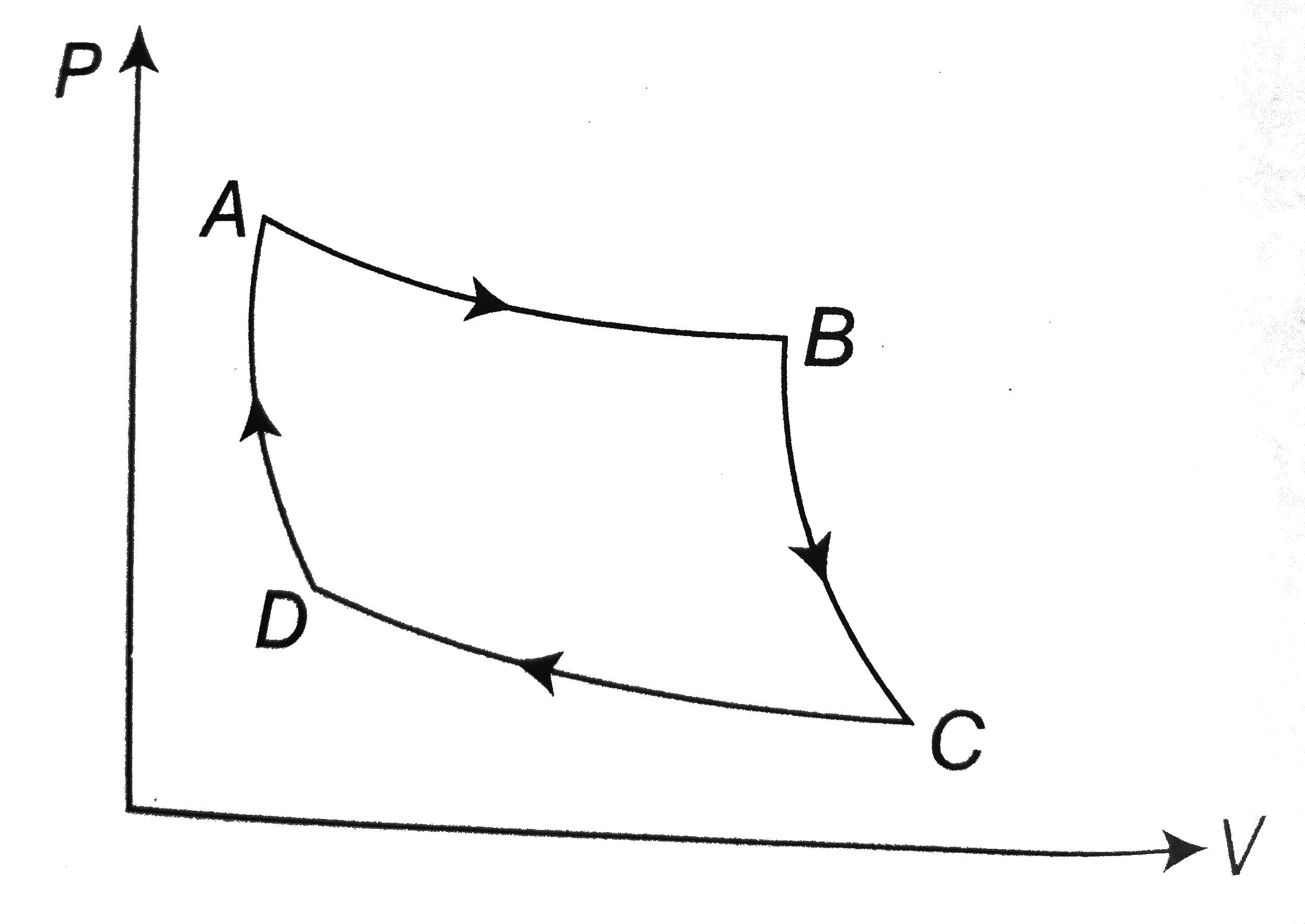
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Problems Based On Mixed Concepts|49 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Assertion Reasoning|15 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Application Of First Law Of Thermodynamics In Different Situations|25 VideosGRAVITATION
A2Z|Exercise Chapter Test|29 VideosMOCK TEST
A2Z|Exercise Motion With Constant Acceleration|15 Videos
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-Second Law Of Thermodynamics
- In a Carnot engine when T(2) = 0^(@)C and T(1) = 200^(@)C its efficien...
Text Solution
|
- A Carnot engine has the same efficiency between 800 K to 500 K and x K...
Text Solution
|
- A Carnot's engine is made to work between 200^(@)C and 0^(@)C first an...
Text Solution
|
- Efficiency of a Carnot engine is 50% when temperature of outlet is 500...
Text Solution
|
- An ideal heat engine working between temperature T(1) and T(2) has an ...
Text Solution
|
- An ideal refrigerator has a freezer at a temperature of -13^(@)C. The ...
Text Solution
|
- In a mechanical refrigerator the low temperature coils are at a temper...
Text Solution
|
- An engine is supposed to operate between two reservoirs at temperature...
Text Solution
|
- An ideal gas heat engine operates in Carnot cycle between 227^(@)C and...
Text Solution
|
- Two Carnot engines are operated in succession. The first engine receiv...
Text Solution
|
- A Carnot engine whose low temperature reservoir is at 7^(@)C has an ef...
Text Solution
|
- Carnot cycle (reversible) of a gas represented by a pressure volume cu...
Text Solution
|
- A motor cycle engine delivers a power of 10 kW, by consuming petrol at...
Text Solution
|
- A heat engine receives 50 kcal of heat from the source per cycle, and ...
Text Solution
|
- A Carnot's engine operates with an efficiency of 40% with its sink at ...
Text Solution
|
- The efficiency of a Carnot cycle is 1//6. By lowering the temperature ...
Text Solution
|
- A Carnot heat engine has an efficiency of 10%. If the same engine is w...
Text Solution
|
- In a cold storage, ice melts at the rate of 2 kg//h when the external ...
Text Solution
|
- A Carnot engine used first an ideal monoatomic gas (gamma = 5//3) and ...
Text Solution
|
- Find the amount of work done to increase the engines ever developed op...
Text Solution
|