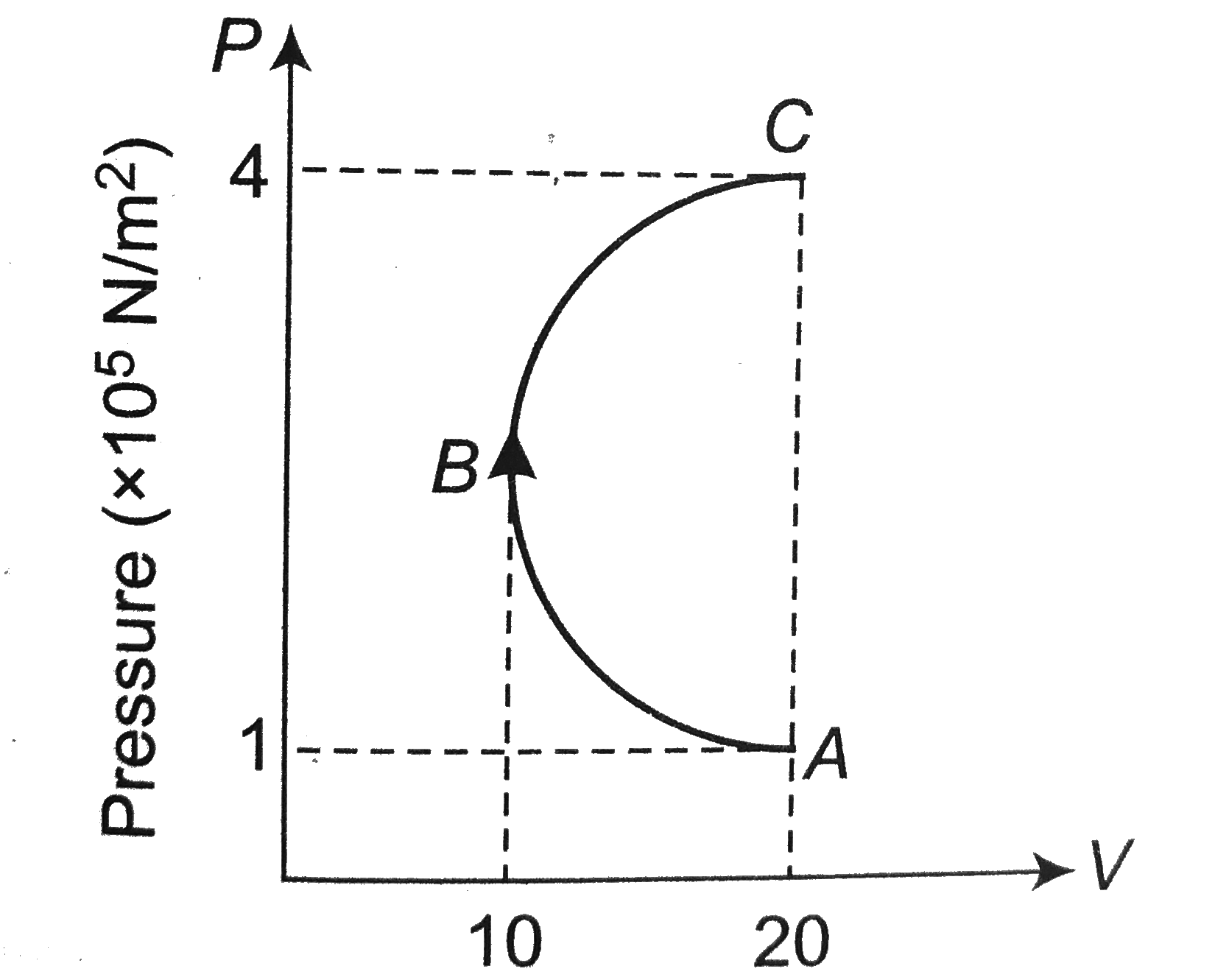
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Assertion Reasoning|15 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise NEET Questions|55 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Second Law Of Thermodynamics|29 VideosGRAVITATION
A2Z|Exercise Chapter Test|29 VideosMOCK TEST
A2Z|Exercise Motion With Constant Acceleration|15 Videos
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-Problems Based On Mixed Concepts
- A gaseous mixture consists of 16g of helium and 16 g of oxygen. The ra...
Text Solution
|
- Suppose a diatomic gas gets ionised to a certain extent without any ex...
Text Solution
|
- A monoatomic ideal gas undergoes a process ABC. The heat given to the ...
Text Solution
|
- A balloon containing an ideal gas has a volume of 10 litre and tempera...
Text Solution
|
- T-V curve of cyclic process is shown below, number of moles of the gas...
Text Solution
|
- P-T curve of a cyclic process is shown. Find out the works done by the...
Text Solution
|
- A sample of an ideal gas has pressure p(0), volume V(0) and tempreture...
Text Solution
|
- Figure shows a vessel partitioned by a fixed diathermic separator. Dif...
Text Solution
|
- The internal energy of a monatomic ideal gas is 1.5 nRT.One mole of he...
Text Solution
|
- A sample of ideal gas (gamma = 1.4) is heated at constant pressure. If...
Text Solution
|
- P-V curve of a diatomic gas is shown in the Fig. Find the total heat g...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- Find the work done by gas going through a cyclic process shown in figu...
Text Solution
|
- In the P-V diagram shown in figure, ABC is a semicircle. Find the work...
Text Solution
|
- A gas has molar heat capacity C = 4.5 R in the process PT = constant. ...
Text Solution
|
- A gaseous mixture enclosed in a vessel consists of one gram mole of a ...
Text Solution
|
- One mole of a gas mixture is heated under constant pressure, and heat ...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process as shown in th...
Text Solution
|
- A gas is undergoing an adiabatic process. At a certain stage A, the va...
Text Solution
|
- The heat supplied to one mole of an ideal monoatomic gas in increasing...
Text Solution
|