A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise AIIMS Questions|27 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Chapter Test|29 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Assertion Reasoning|15 VideosGRAVITATION
A2Z|Exercise Chapter Test|29 VideosMOCK TEST
A2Z|Exercise Motion With Constant Acceleration|15 Videos
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-NEET Questions
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- The ratio of the specific heats (C(P))/(C(upsilon)) = gamma in terms o...
Text Solution
|
- Two vessel separately contains two ideal gases A and B at the same tem...
Text Solution
|
- 4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity...
Text Solution
|
- The coefficient of performance of a refrigerator is 5. If the temperat...
Text Solution
|
- An ideal gas is compressed to half its initial volume by means of seve...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- The molecules of a given mass of a gas have rms velocity of 200 m//s a...
Text Solution
|
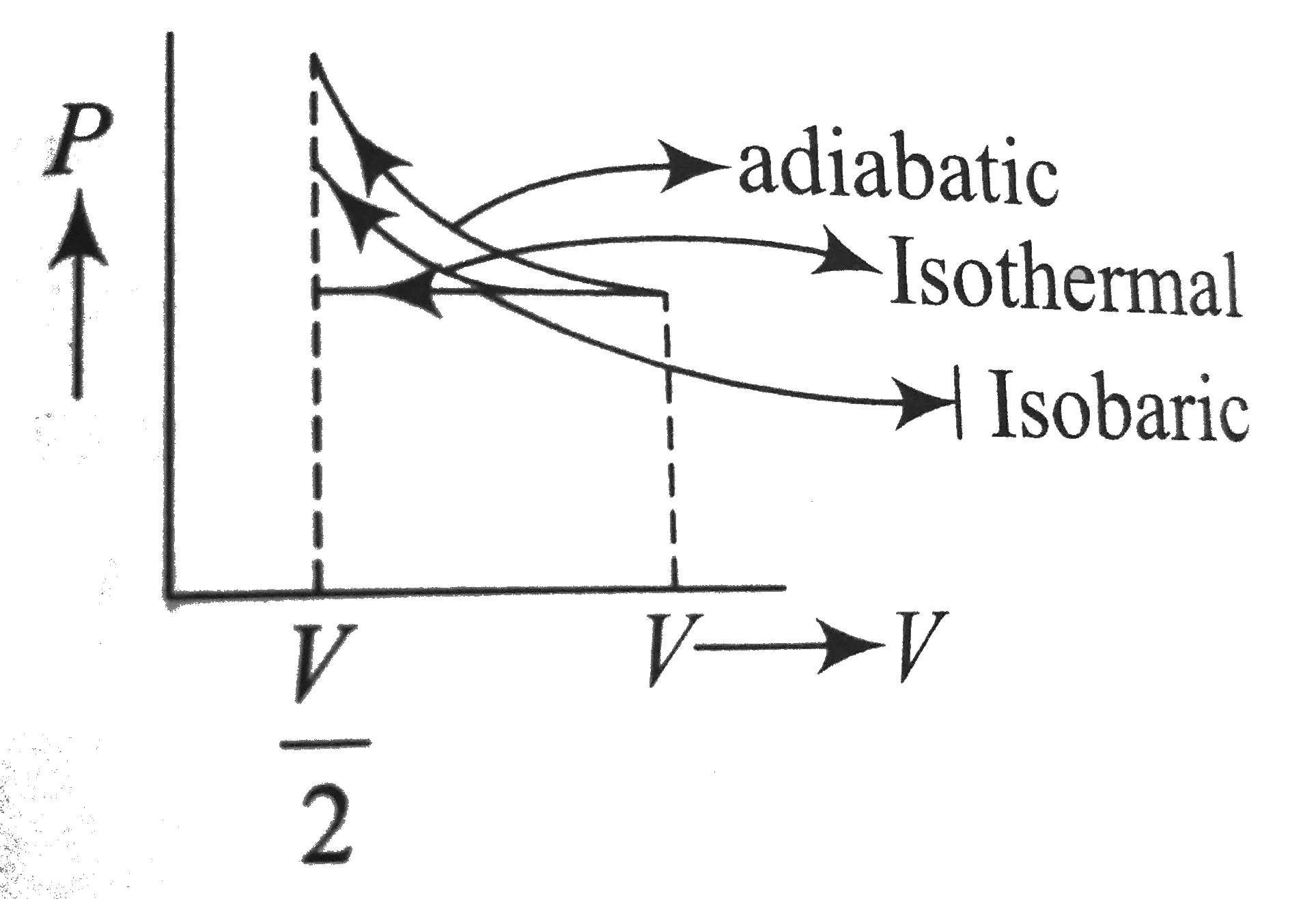
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- One mole of an ideal monatomic gas undergoes a process described by th...
Text Solution
|
- The temperature inside a refrigerator is t(2)^(@)C . The amount of hea...
Text Solution
|
- A sample of a perfect gas occupies a volume V at a pressure P and obso...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|
- At what temperature , will the rms speed of oxygen molecules be suffic...
Text Solution
|
- The efflciency of an ideal heat engine working between the freezing p...
Text Solution
|
- The volume (V) of a manatomic gas varies with its temperature (T) , as...
Text Solution
|
- A sample of 0.1 g of water of 100^(@)C and normal pressure (1.013 xx 1...
Text Solution
|