A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-AIIMS Questions
- A gas is enclosed in a closed pot. On keeping this pot in a train movi...
Text Solution
|
- Two ballons are filled, one with pure He gas and other by air, repecti...
Text Solution
|
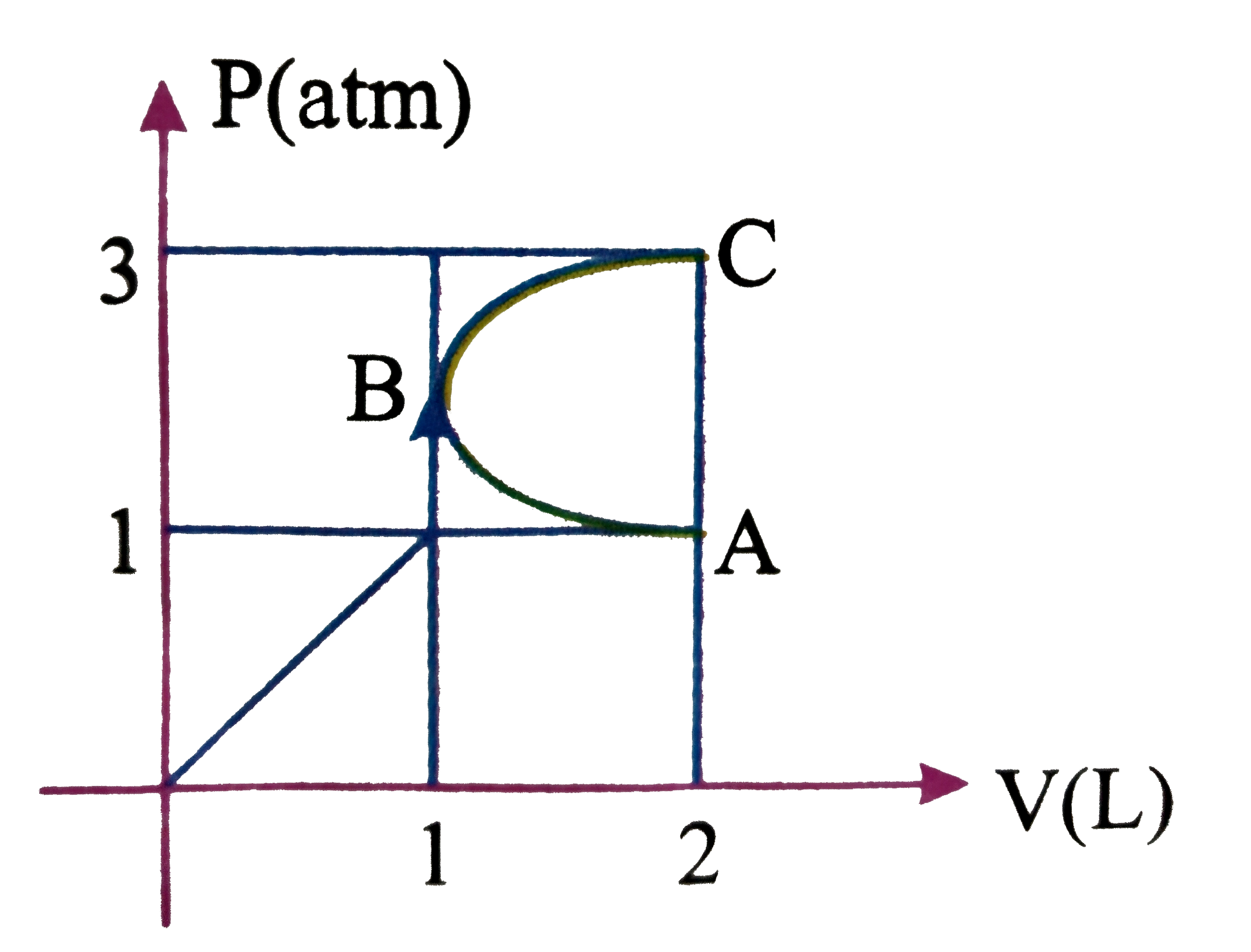
- In the P-V diagram shown shown in figure ABC is a semicircle.The work ...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- A gas is compressed adiabatically till its temperature is doubled. The...
Text Solution
|
- A reversible engine converts one-sixth of the heat input into work. Wh...
Text Solution
|
- A gas expands adiabatically at constant pressure such that its tempera...
Text Solution
|
- A system is provided with 200 cal of heat and the work done by the sys...
Text Solution
|
- Two cylinder having m(1)g and m(2)g of a gas at pressure P(1) and P(2...
Text Solution
|
- When an ideal monoatomic gas is heated at constant pressure, fraction ...
Text Solution
|
- A carnot engine operates with source at 127^(@)C and sink at 27^(@)C. ...
Text Solution
|
- Assertion: In adiabatic compression, the internal energy and temperatu...
Text Solution
|
- Assertion: When a bottle of cold carbonated drink is opened, a slight...
Text Solution
|
- Aseertion: Thermodynamics process in nature are irreversible. Reason...
Text Solution
|
- Assertion: Reversible systems are difficult to find in real world. R...
Text Solution
|
- Assertion: Air quickly leaking out of a balloon becomes coolers. Rea...
Text Solution
|
- Assertion: In an isolated system the entropy increases. Reason: The ...
Text Solution
|
- Assertion: The carnot cycle is useful in understanding the performance...
Text Solution
|
- Assertion: The isothermal curves intersect each other at a certain poi...
Text Solution
|
- Assertion: The heat supplied to a system is always equal to the increa...
Text Solution
|