A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-Chapter Test
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
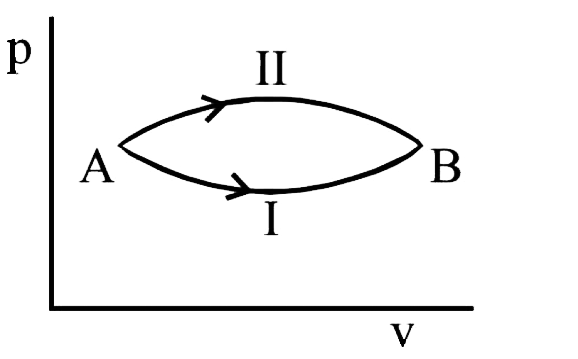
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- Two identical containers A and B with frictionless pistons contain the...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- In a given process on an ideal gas, dW=0 and dQlt0. Then for the gas
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- An ideal gas is filled in a closed rigid and thermally insulated conta...
Text Solution
|
- A Carnot engine operates between 327^(@)C and 27^(@)C How much heat (i...
Text Solution
|
- One mole of gas having gamma = 7//5 is mixed with 1 mole of a gas havi...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- Internal energy of n(1) mol of hydrogen of temperature T is equal to t...
Text Solution
|
- An ideal gas (gamma = 1.5) is expanded adiabatically. How many times h...
Text Solution
|