Text Solution
Verified by Experts
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise EXERCISES|29 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise linked Comprehension Type|38 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Single correct Answer|14 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
CENGAGE CHEMISTRY-ALKENES AND ALKADIENES-SOLVED Example
- What is the numberical value of (B) and (D) and what types of isomers...
Text Solution
|
- Complete the following reaction :
Text Solution
|
- Complete the following reaction :
Text Solution
|
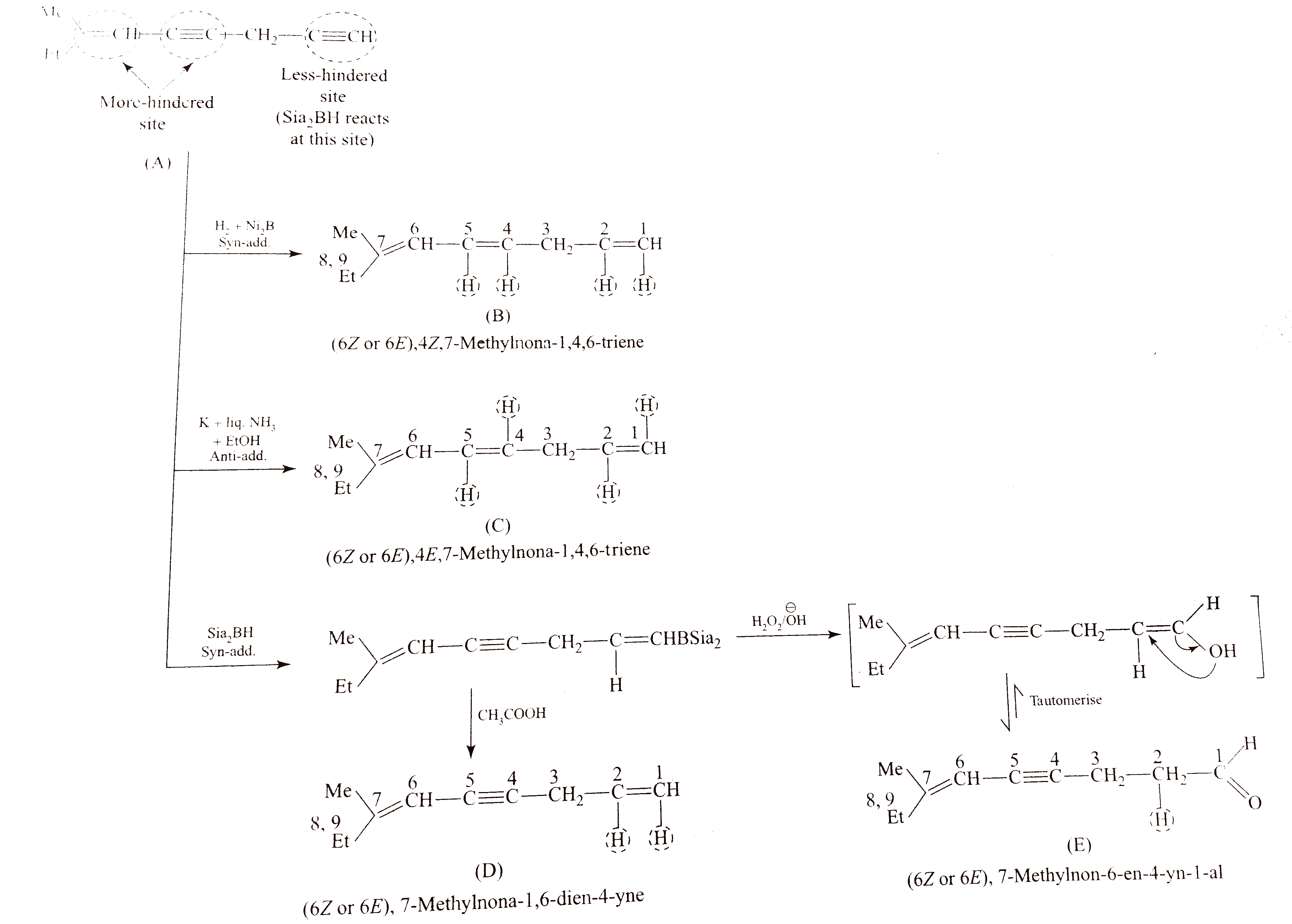
- Identify (A) to (H).
Text Solution
|
- Write the product with its mechanism.
Text Solution
|
- Identify (A) to (D).
Text Solution
|
- Identify (A) to (G).
Text Solution
|
- Bulky t-butyl group is always on the equatorial position because of ...
Text Solution
|
- Which of the following show G.I.? a. b. c. d. e. ...
Text Solution
|
- Give IUPAC name to each to the following using E or Z designations. ...
Text Solution
|
- Give IUPAC names for the following using E and Z designations. a. ...
Text Solution
|
- Give IUPAC name of the following using E//Z and R//S designations. a...
Text Solution
|
- Complete the following reaction. a. b. Alcohols are converted to...
Text Solution
|
- Give the reaction of the following : i. ii. 2-Bromo butane + C(2...
Text Solution
|
- Give the decreasing order or reactivity for the elimination with stron...
Text Solution
|
- A compound (A) is a sodium salt of dibasic acid and on electrolysis gi...
Text Solution
|
- Give the structure of an optically active alkene (A) having the lowest...
Text Solution
|
- Give the structure of an optically active alkyne (A) having the lowest...
Text Solution
|
- Give the structure of an optically active unsaturated hydrocarbon (A) ...
Text Solution
|
- Explain the formation of (A) and (B) in the following reaction. Oct-...
Text Solution
|
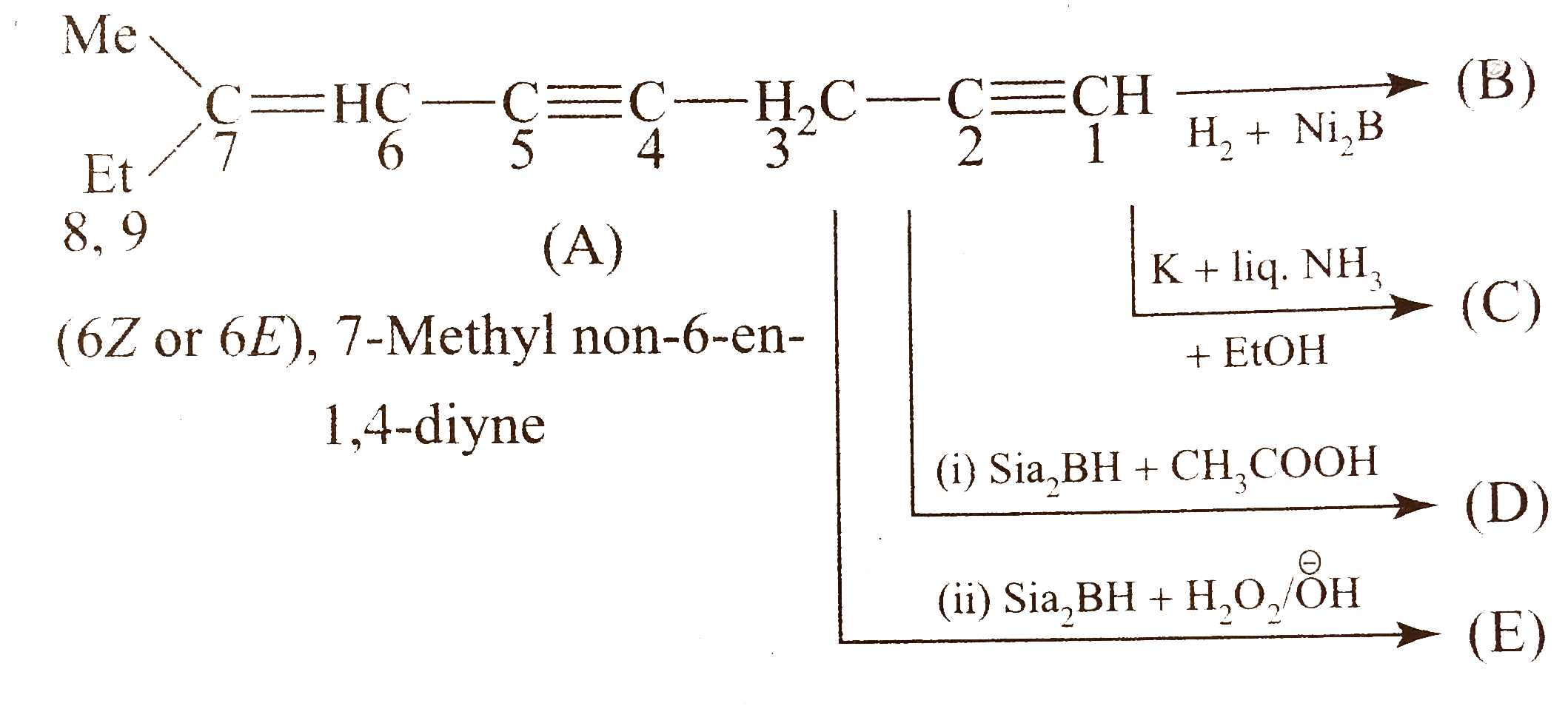
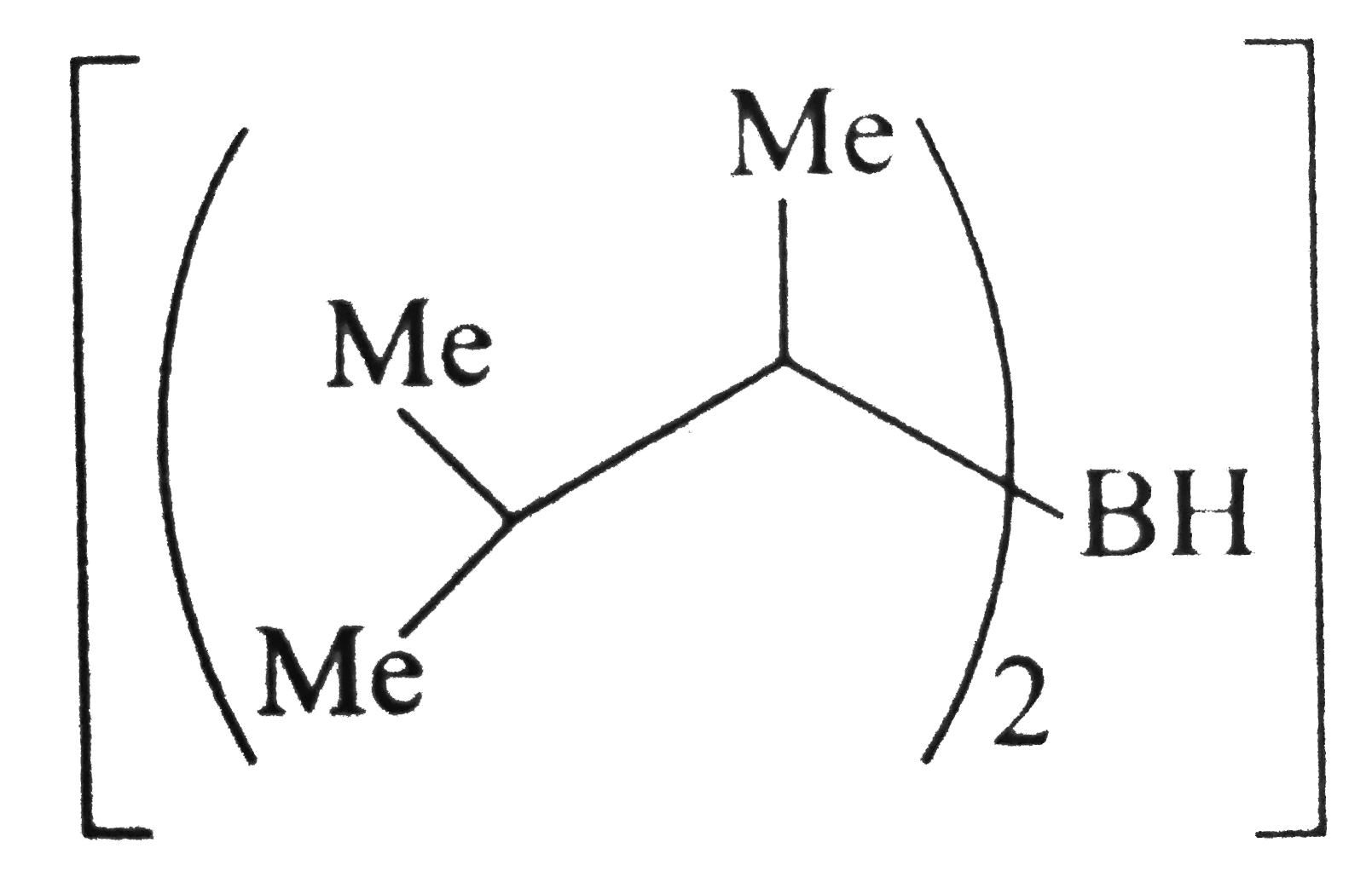 is a sterically hindered dialkyl borane and reduces less `-` hindered `(C-=C)` to `(C=C)` and less `-` hindered `(C=C)` to `(C-C)` in the presence of `CH_(3)COOH`, but with `H_(2)O_(2)//O^(Θ)H` it gives anti`-` Markovnikov's products `(` alcohols `)` that tautomerise to give aldehydes or ketones.
is a sterically hindered dialkyl borane and reduces less `-` hindered `(C-=C)` to `(C=C)` and less `-` hindered `(C=C)` to `(C-C)` in the presence of `CH_(3)COOH`, but with `H_(2)O_(2)//O^(Θ)H` it gives anti`-` Markovnikov's products `(` alcohols `)` that tautomerise to give aldehydes or ketones.